Optimizing the AAV Transfection Process in Suspension CellsOptimizing the AAV Transfection Process in Suspension Cells
Adenoassociated virus (AAV) is ideal for gene therapy delivery because it is nonpathogenic, exhibits low immunogenicity, and readily enters several cell types. https://www.stock.adobe.com
Given the potentially curative nature of gene and gene-modified cell therapies and the successful launches of several such products over the past five years, markets and investments are growing significantly in the sector. In the United States alone, the US Food and Drug Administration has granted approval for several genetic therapies, including
Strimvelis (autologous CD34+, developed by GlaxoSmithKline and later sold to Orchard Therapeutics) in 2016
Luxturna (voretigene neparvovec, Spark Therapeutics), Yescarta (axicabtagene ciloleucel, Kite Pharma/Gilead), and Kymriah (tisagenlecleucel, Novartis) in 2017
Zolgensma (onasemnogene abeparvovec, AveXis/Novartis) in 2019
Tecartus (brexucabtagene autoleucel, Kite/Gilead) in 2020.
Investments in gene therapy companies increased by 70% in 2020 (1), and at the beginning of 2021, 423 clinical trials were underway for candidate gene therapies, with roughly half of those in phase 2 studies. That includes candidate treatments for eye diseases, infection by human immunodeficiency virus (HIV), sickle cell disease, cystic fibrosis, congestive heart failure, hemophilia, cancer, and several other genetic disorders (2).
Nearly 70% of approved and pipeline gene therapies rely on viral-vector delivery, typically adenoassociated virus (AAV) for in vivo and lentivirus (LV) for ex vivo applications (3). Developers often use the former virus for gene transfer because it is nonpathogenic, exhibits low immunogenicity, and readily enters several cell types (4).
Serious Constraints Abound
The rapid advance of many gene therapy candidates is raising several obstacles. In addition to capacity shortages for both raw material (plasmid DNA) and viral-vector production, developers and manufacturers need to make significant improvements to the efficiency, productivity, and scalability of upstream and downstream viral-vector processes. Such advances are essential to reducing the cost of gene and gene-modified cell therapies. For instance, AAV-based gene therapies carry some of the highest prices ever seen for drug treatments: US$850,000 and $2.1 million for the Luxturna and Zolgensma products, respectively.
AAV vectors are expressed in cell culture, then purified chromatographically. In many cases, capture steps are followed by ultracentrifugation or anion-exchange chromatography to reduce partially filled and empty viral capsids. Tangential-flow filtration (TFF) is used to transfer vectors into a desired buffer solution. That is subjected to sterile filtration and aseptic filling.
Currently, most vector production systems show low yields. Compounding that problem is low recovery after chromatographic polishing. The greatest opportunity for process improvement lies upstream because titer and yield increases of several logs could augment overall process yields significantly. Downstream, the goal is to increase vector recovery by twofold. Currently, that ranges from 40% to 50% (5).
Triple Transfection Predominates
The most common method for producing recombinant AAV (rAAV) is transient transfection of adherent or suspension-adapted human embryonic kidney (HEK)293 cells using three plasmids and a transfection agent. One plasmid contains a gene of interest (GoI) flanked by inverted terminal repeats (ITRs) that enable cellular infection and subsequent expression of the AAV genome and replicate. A second plasmid contains the AAV replicative (rep) and capsid (cap). A helper plasmid contains several open reading frames of the adenovirus genome.
Less-common vector production methods include infection of baculovirus or herpes simplex virus. Some manufacturers have begun to use stable producer and packaging cell lines, but that approach requires significantly more upfront development time, and difficulties remain for control of viral protein production to prevent cell toxicity (6).
HEK293 cell transfection predominates because it offers flexibility and robustness. The cell line is well established in the biopharmaceutical industry. Triple transfection has been used since the late 1990s, uses natural AAV sequences, and enables rapid exchanging of GoIs and serotype sequences. Therapy developers and manufacturers also can leverage serum-free suspension cell cultures for such processes.
Optimizing Plasmids
LogicBio Therapeutics, Inc., is a clinical-stage genetic medicine company investigating gene-editing and gene-delivery platforms to address rare and serious diseases from infancy through adulthood. Using its proprietary GeneRide editing platform for precise gene insertion, the company aims to harness cells’ natural DNA repair processes, potentially leading to durable therapeutic-protein expression levels. Aligned with the proprietary sAAVy gene delivery platform, AAV capsids are engineered to optimize gene transfer to a broad range of tissue types and for many indications. All that is performed using applied theories of directed evolution, rational design, and machine learning. Resulting capsid variants are screened in clinically predictive models such as humanized mice and human tissue explants to guide selection of the most effective vectors.
To improve the manufacturability of sAAVy capsids and overall cost-effectiveness of vector manufacturing, LogicBio has optimized plasmids for transient transfection of HEK293 cells. The plasmids maximize AAV capsid production while preventing generation of replication-competent AAVs (rcAAVs) that can develop through plasmid recombination. The plasmids also retain the flexibility of the original transfection system and show good manufacturing yields.
In LogicBio’s experience, transfection of suspension HEK293 cells using a commercially available polyethyleneimine (PEI) transfection agent can enable two- to fivefold increases in vector titer for several capsid variants over levels achievable using standard three-plasmid systems. Even at the highest vector dosage (1010 viral genomes (vg)/mL), LogicBio has detected no rcAAV in the resulting vector preparations.
Using Fit-for-Purpose Reagents
In a triple transfection process, the transfection agent can influence capsid-formation efficiency significantly. LogicBio has investigated reagents designed specifically for AAV transfection with its proprietary plasmids. To optimize rAAV yields and improve process scalability for clinical and commercial use, the company ultimately used materials from the FectoVIR-AAV chemical-based, animal-component–free transfection reagent portfolio engineered by Polyplus-transfection.
LogicBio optimized its transfection parameters using a multifactor design-of-experiments (DoE) approach. Parameters were tested in a three-factor DoE assessment. Then, the two most critical parameters were refined in a two-factor study. The optimized conditions were confirmed in bioreactors, which generated high volumetric yields in crude harvests. A Droplet Digital polymerase chain reaction (ddPCR) titration assay (from Bio-Rad Laboratories) targeting the GoI showed yields ranging from 5 × 1011 vg/mL to 8 × 1011 vg/mL.
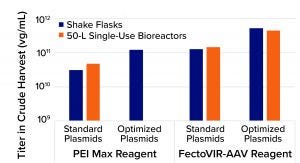
Figure 1: Results from adenoassociated virus (AAV) transfection processes using standard plasmids, polyethylenimine (PEI), optimized plasmids developed by LogicBio Therapeutics, and/or FectoVIR-AAV transfection regent from Polyplus-transfection
Compared with standard triple transfection using commercially available PEI, the combination of LogicBio plasmids and FectoVIR-AAV reagent increased titers 10- to 20-fold. In all cases, the optimized plasmids enabled three- to fivefold yield increases; the AAV-focused transfection brought additional three- to fivefold improvements. Equally important is that the >10-fold yield improvements were achieved when the optimized transfection process was scaled from shake flasks to 50-L single-use bioreactors. Analytical characterization confirmed that the capsid quality attributes (e.g., viral protein ratios) were comparable between processes using standard plasmids with PEI and using optimized plasmids with FectoVIR-AAV reagent (Figure 1).
Bolstering Vector Production
Transient transfection has become the gold standard for producing AAV vectors needed to deliver gene therapies. The current process using standard plasmids and general transfection reagents presents significant opportunities for improving vector titers and yields. LogicBio has developed plasmids that can generate high yields of AAV capsids. Applying those plasmids with FectoVIR-AAV transfection agent can boost AAV vector yields in HEK293 suspension cells significantly. The new process’s conditions are scalable and maintain comparable vector quality attributes. Because these raw materials enable >10-fold improvements in yields, they could enable cost-effective clinical and commercial manufacture of AAV vectors and ultimately help reduce the expected per-patient cost of future gene therapies.
References
1 2020: Growth and Resilience in Regenerative Medicine. Alliance for Regenerative Medicine: Washington, DC, 2021; http://alliancerm.org/wp-content/uploads/2021/03/ARM_AR2020_FINAL-PDF.pdf.
2 Finkel E. The Gene Therapy Revolution Is Here — Medicine Is Scrambling to Keep Pace. The Conversation 4 June 2019; https://theconversation.com/the-gene-therapy-revolution-is-here-medicine-is-scrambling-to-keep-pace-118329.
3 Micklus A. Gene Therapy: A Paradigm Shift in Medicine. Pharm. Intell., November 2018; https://pharmaintelligence.informa.com/~/media/informa-shop-window/pharma/whitepapers/dmhc-gene-therapy-whitepaper.pdf.
4 Darling S. Facilitating Gene Therapy Development with Solutions to Four Capsid Analytical Challenges. Cell Gene Ther. Insights 7(3) 2021: 411–415; https://doi.org/10.18609/cgti.2021.067.
5 Erbacher P. Are Suspension-Based Systems for Cell and Gene Therapy Key to Commercial-Scale Manufacture? Cell and Gene Ther. Insights 6(3) 2020: 221–226; https://doi.org/10.18609/cgti.2020.029.
6 Danby S. Overcoming AAV Manufacturing Challenges. Contract Pharm. 5 May 2021; https://www.contractpharma.com/issues/2021-05-01/view_features/overcoming-aav-manufacturing-challenges.
Matthias Hebben, PhD, is vice president and head of technology development at LogicBio Therapeutics, 65 Hayden Ave, Lexington, MA 02421. Alengo Nyamay’antu, PhD, is a scientific communication specialist at Polyplus-transfection SA, VECTURA,
75 Rue Marguerite Perey, 67400 Illkirch-Graffenstaden, France.
You May Also Like






