Human Interferon Beta-1b Production in Pseudomonas fluorescensHuman Interferon Beta-1b Production in Pseudomonas fluorescens
July 1, 2010

Interferon beta-1b is a protein in the interferon family used to treat the relapsing-remitting form of multiple sclerosis (MS). Interferon beta-1b is a natural human protein (molecular weight ~18,500 Da) that is produced in the body in response to viral infection and has antiviral activity. It has been shown to slow the advance of MS and reduce the frequency of attacks. It is believed that interferon-beta achieves this effect on MS progress via its anti-inflammatory properties. interferon beta-1b drugs have been approved for over 20 years to treat the symptoms of MS. interferon beta-1b is produced as a recombinant protein in the bacterium E. coli by fermentation and subsequent purification to the active drug. The E. coli bacteria produce the interferon beta-1b molecule at low yield and as an insoluble and inactive product. As part of the purification process, the molecule must be restored to its active state, a process known as refolding. Refolding a protein molecule is a difficult, inefficient, and costly process. If interferon beta-1b could be produced by a bacterium at high yield in a fully active form without the necessity of refolding, it would be much less expensive to produce and could be offered to patients at a more affordable price.
The Pseudomonas fluorescens-based Pfēnex Expression Technology platform is a proven high-throughput, parallel screening, protein expression strain development platform that has been designed specifically to deliver bacterial production strains expressing high yields of soluble and active protein. It is based on an extensive toolbox of expression strategies that can be seamlessly combined to deliver robust expression strains for the production of recombinant proteins. Scientists at Pfēnex Inc. — a San Diego, Ca–based protein discovery, development, and production company — have cloned and expressed many difficult-to-express proteins in their soluble and active forms and successfully completed scale-up for manufacturing. Pfēnex scientists, using the platform, cloned the interferon beta-1b– coding gene into 20 unique expression plasmids and transformed them into 30 phenotypically distinct P. fluorescens host strains. The resulting 600 expression strains were grown in 96-well plates, and the expression of interferon beta-1b was determined for each strain, all in about one month.
Subsequently identified, high-expressing strains were tested in scaled-down fermentation vessels (at 4 ml and then 1 L scales) using design-of-experiment tools under multiple fermentation conditions. The results of these experiments identified an interferon beta-1b production strain and fermentation conditions yielding 8 g/l of soluble, active interferon beta-1b. A downstream recovery and purification process has been developed yielding highly purified and fully characterized interferon beta-1b (Figure 1).This process is cGMP-ready. Pfēnex Inc. is now offering for sale reagent-grade recombinant interferon beta-1b in small lots and seeking a partner to develop the molecule for human therapeutic use.
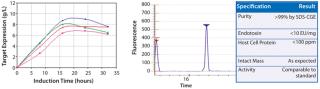
Figure 1: ()
About the Author
Author Details
Charles H. Squires, PhD, is vice president of discovery and partnerships for Pfēnex Inc., 5501 Oberlin Drive, San Diego, CA 92121; 1-858-352-4400, fax 1-858-352-4602; [email protected], www.pfenex.com.
You May Also Like






