Bacteriophages, an Alternative to Antibiotics: Challenges and Possible Solutions for Bringing Them to MarketBacteriophages, an Alternative to Antibiotics: Challenges and Possible Solutions for Bringing Them to Market
June 16, 2016
Concept of attack. Bacteriophage are viruses (with genetic material) that infect bacteria.
Bacteriophages are viruses (consisting of a genome contained within a protein coat) that specifically infect bacteria. They are the most abundant living entities on earth — the estimates range from 1030 to 1032 in total — and play key roles in regulating the microbial balance in every ecosystem where that has been explored (1). Bacteriophages are genotypic and phenotypically different from viruses that infect Archaea (Archaeovirus) and Eukarya (Eukaryovirus). The name bacteriovirus has been proposed as scientifically more accurate (2). Bacteriophage classification has been assigned to the International Committee for Taxonomy of Viruses (ICTV). It organized bacteriophages into 13 different families of the Caudovirales order (3).
Once a bacteriophage encounters a target bacterium, the process of bacteriophage replication takes place. The bacteriophage adsorbs and ligates to the bacterial membrane surface receptors. Then the sheath contracts, and a hollow-fiber tail penetrates through the cell membrane, injecting bacteriophage nucleic acid into the bacterial periplasm. The genetic material of the bacteriophage takes up the biosynthetic machinery of the host, and during an “eclipse” period, mRNA expression occurs, resulting in directed macromolecular biosynthesis. During maturation, the previously synthesized bacteriophage structural proteins are assembled, and bacteriophage particles accumulate inside the cell. At the end of a latent period, accumulation of lytic proteins causes cell lysis and release of bacteriophages. The burst size corresponds to the average number of progeny bacteriophage particles produced per infected bacterium.
Treating bacterial infections with phages differs from traditional topical or systemic antibiotic therapies as bacteriophages replicate and thus increase in concentration at the site of infection. Antibiotics decrease in concentration as they are dispersed into the tissues.
History
Bacteriophages were discovered in 1915 by British microbiologist Felix Twort and independently in 1917 by French-Canadian microbiologist Felix d’Hérelle. Although Twort did not pursue his discovery, d’Hérelle systematically investigated the nature of bacteriophages and explored their ability to function as therapeutic agents. Microbiologists subsequently began to study bacteriophages and the potential of phage therapy as a treatment (4).
After World War II, interest in phages in Western countries declined rapidly with the rise of antibiotics. This was not the case in the Soviet Union — especially Georgia, where in 1930 the Eliava Institute in Tbilisi was founded. The Eliava Institute subsequently developed a large manufacturing operation (production capacity of ∼2 tons/week), which employed 1,200 people, most of whom were involved in phage production. The bulk of their output was shipped to the Soviet military, predominantly for the treatment of diarrhea and wounds, and the rest was available in different forms to the general public.
The potential therapeutic use of bacteriophages has undergone renewed interest in recent years because of increasing difficulties in curing infections caused by antibiotic-resistant bacterial strains and by increasing knowledge of the structure and function of phages. The latter includes the possibility of characterizing phages genetically by sequencing their entire genomes. Proteins derived from phages also are being investigated as antimicrobial agents (5).
Bacteriophage treatment is currently not accepted by mainstream medicine because of a lack of robust scientific evidence emanating from well-designed controlled good clinical practice (GCP) trials. Scientific knowledge regarding the biology, genetics, and bactericidal efficacy of bacteriophages in vitro is extensive, but considerably less is known about in vivo behavior, particularly in human bodies.
To develop robust, reliable data and establish the safety and efficacy profile of bacteriophage therapy, a welldesigned clinical development plan with GCP-regulated clinical trials will be required.
Regulatory Framework
One major challenge to the clinical application of bacteriophages in Western medicine is adapting the regulatory framework to appropriately reflect the novel mode of action of these unusual antimicrobials — being both self-replicating and self-limiting. As described above, bacteriophage products have already been marketed in some Eastern European countries, including Poland Poland (a member of the European Union). Those markets found short term, interim solutions to the regulatory challenge. They considered bacteriophage therapy as an experimental treatment within the responsibility and supervision of medical ethical committees, covered by the Physician Practice Act (Polish Law Gazette Number 28 of 1997) and World Medical Association Declaration of Helsinki (6–8). However, provisional solutions are not substitutes for fully controlled clinical trials in accordance with the United States Food and Drug Administration (FDA) and the European Medicines Agency (EMA) regulatory framework.
The EMA has placed bacteriophages under medicinal product regulation, specifically under the regulatory framework for biological medicinal products. At the FDA, bacteriophage applications are handled by the Division of Vaccines and Related Product Applications part of the Center for Biologics Evaluation and Research (CBER). However, neither guideline fully covers aspects specific to bacteriophages. Therapies are usually administered in fixed phage cocktails, so questions arise about what to consider as the drug substance and drug product.
A drug product would usually be the final formulation consisting of a variety of phages (usually in a topical form). Drug substances would be the individual phages forming the cocktail. A drug substance could be defined at the level of the phage strain — not at the level of the individual phage — to provide for flexibility in replacing one phage with another phage from the same strain.
The composition of such phage cocktails would require some flexibility, so that (in case of resistance) they could be adapted quickly. Under current regulations, it is impossible to change the composition of a marketed drug without having to perform an extensive program of additional studies (clinical and nonclinical). This is a major challenge to the development of phages as a treatment — the ability to rapidly update the formulation or cocktail of phages being essential to preserve efficacy.
To promote research and development in this field, the EMA and FDA may have to revise or update their rules as for seasonal influenza vaccines, which also require rapid updating and licensing procedures. That approach raises another problem, however, because even if a change in the cocktail could be submitted as a type II variation, the current regulatory framework would not allow for two different cocktails to be marketed at the same time.
Such regulatory hurdles and lack of strong intellectual property protection have hampered pharmaceutical companies in marketing bacteriophage preparations. To avoid the drug-licensing pathway, some US companies decided to first develop bacteriophage products for the decontamination of food, plants, fields, and livestock (10). Currently, the FDA and the US Department of Agriculture, Food Safety, and Inspection Services (FSIS) regard commercial bacteriophage preparations as safe and approved for use in food consumed by humans (71 Fed. Reg. 47729, 2006).
On 8 June 2015, the EMA organized a workshop on therapeutic use of bacteriophages (EMA/389257/2015). Its objective was to to bring together relevant stakeholders (including academia, industry, policy makers, and patient organizations) to proactively discuss possible issues related to development of bacteriophage therapies for treating bacterial infections.
The EMA has stated that it maintains a position of openness toward exploring all possibilities for using current regulatory frameworks to allow further development of this therapeutic approach. Yet at the workshop, it was emphasized that a medicine cannot be recommended for market approval before its efficacy and safety have been proven on the basis of appropriately designed clinical trials. That is not currently the case for bacteriophage therapies, for which very few randomized controlled clinical trials have been conducted to date.
Manufacturing
Although current medicinal product regulations do not provide specific guidelines for therapeutic bacteriophage products (6, 7), existing quality requirements for biotechnological products (11) can serve as a basis. In addition, quality guidelines for live viral vaccines can be used as a support.
The first step is isolation, identification, and choice of the phages. That choice relies on one of two models. The first involves cocktails of multiple phages that display a wider spectrum of activity than their individual phage components. That approach allows for use against a wider range of bacterial targets and virtually eliminates resistance developing in the short term. For the second approach, pathogenic bacteria are isolated from infections and tested against a large, generally well-characterized collection of previously isolated phages.
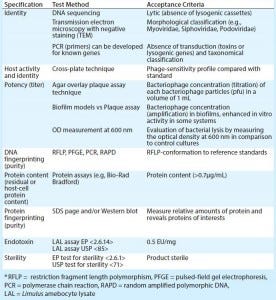
Table 1: Quality parameters of bacteriophage drug substances and assessment methods (12)
Irrespective of whether a therapeutic product is a single phage or a phage cocktail, all individual components must be genomically and phenotypically characterized. That ensures that a preparation will contain only well-defined bacteriophages. The morphological types of bacteriophages should be identified using the characterization techniques summarized in Table 1.
An expert consensus on the quality and safety requirements for sustainable phage-therapy products has been established (13). It describes known risks and tests that can be performed to minimize those risks.
Manufacturing processes of phages will need to be carried out according to good manufacturing practice (GMP). And they must be described fully in chemistry, manufacturing, and controls (CMC) documentation as part of an investigational new drug (IND) or clinical trial application (CTA) submission.
Clinical Development
The clinical development of bacteriophages is similar to other products. However, it has some unique considerations. That is particularly attributed to the self-replicating ability of bacteriophages, which will influence trial design (14).
Researchers believe that 90% of phages are suitable for phage therapy (15). To be considered as therapeutic agents, phages must have specific abilities to infect, lyse, and kill bacteria. A suitable phage must be strictly virulent and be able to reproduce rapidly and effectively. And therapeutic phages must have a high burst size — one infected bacterium giving rise to a large number of progeny phages released from one infected bacterial cell. But even if phages exhibit a broad host range, it is very difficult to achieve complete coverage of all pathogenic bacteria.
Phages that can lysogenize (become integrated into the genome of a bacterium) are unsuitable for use in therapy. Such temperate phages pose a risk because they potentially can transduce resistance genes from one bacterium to another. Paradoxically, a therapy using such phages could contribute to the spread of antibiotic resistance among bacteria.
Evolution of bacterial resistance to a particular phage (just as to an antibiotic) is inevitable, even if the dynamics of bacterial resistance toward antibiotics may differ (16). Bacterial resistance to phages typically involves just a single-point mutation that changes the bacterial surface antigen needed for phage adhesion. Such mutations happen quite frequently and may be expected to happen during phage therapy (17). A solution to this problem could be to use phages with fast adsorption rates and large burst sizes. Such therapies might rapidly lyse large bacterial bacterial populations, reducing numbers to such low numbers that resistance becomes unlikely.
The most common solution adopted by proponents of phage therapy has been to use a cocktail of several phages, each having tested virulent against a target bacterial strain and that bind on a different surface receptor. This, in theory, makes it very difficult for bacteria to develop resistance against that cocktail. Most clinical trials performed until now have used cocktails of phages.
Administration of large amounts of phages has led to no immunological complications (18). Previous clinical and animal trials have also demonstrated no serious immunologic reactions (19).
Typically, bacteriophages are administered topically and used as an active (self-replicating) treatment. Conventional pharmacokinetic (PK) investigational methodologies may not be applicable, given that phages increase in concentration relative to bacterial load and do not conform to usual ADME principles. A bacteriophage’s concentration at an infection site, its bodily distribution, and its rate of clearance (20) after application are crucial factors to consider when designing a dose schedule.
Part of the Solution to Antibiotic Resistance
Phage therapy has had a long history. But for most of that history, the relative benefits of this biological control has been neglected by Western healthcare systems., where conventional antibiotics have been the standard antimicrobial treatment for decades. Concerns regarding the serious threat of antibiotic resistance to populations — recently stressed by the World Health Organization (21) — have led to renewed interest supporting further development of bacteriophages as a medicinal treatment. A number of commercial companies have been established with the aim of further developing bacteriophages for a broad set of indications.
Phage therapy most likely will never totally replace conventional antibiotic treatment, but the option now seems to be developing for a strategy that could provide a complementary approach to treat infections. It should be practicably possible to apply large enough doses of phages and risk of immunological complications.
Several regulatory hurdles might require to be prevented in the clinical development of a bacteriophage therapy. But a closer collaboration between industry and regulatory agencies should be able to bridge the gaps and find mutually acceptable solutions to overcome those barriers.
References
1 Abedon ST. Phage Evolution and Ecology. Adv. Appl. Microbiol. 67, 2009: 1–45; doi:10.1016/S0065-2164(08)01001-0.
2 Raoult D, Forterre P. Redefining Viruses: Lessons from Mimivirus. Nat. Rev. Microbiol. 6(4) 2008: 315–319; doi:10.1038/nrmicro1858. Epub 2008 Mar 3.
3 Ackermann HW. Phage Classification and Characterization. Methods Mol. Biol. 501, 2009: 127–140; doi:10.1007/978-1-60327-164-6_13.
4 Abedon ST, et al. Phage Treatment of Human Infections. Bacteriophage 1(2) 2011: 66–85.
5 Briers Y, Lavigne R. Breaking Barriers: Expansion of the Use of Endolysins As Novel Antibacterials Against Gram-Negative Bacteria. Future Microbiol. 10(3) 2015: 377–390.
6 Verbeken G, et al. European Regulatory Conundrum of Phage Therapy. Future Microbiol. 2(5) 2007: 485–491.
7 Verbeken G, et al. Call for a Dedicated European Legal Framework for Bacteriophage Therapy. Arch. Immunol. Ther. Exp. 62, 2014: 117–129; doi:10.1007/s00005-014-0269-y. Epub 2014 Feb 6.
8 World Medical Association General Assembly 59, Seoul, Republic of Korea, 2008.
9 Wood JM, Levandowski RA. The Influenza Vaccine Licensing Process. Vaccine 21(16) 2003: 1786–1788.
10 Thiel K. Old Dogma, New Tricks: 21st Century Phage Therapy. Nat. Biotechnol. 22(1) 2004: 31–36.
11 EMA/CHMP/BWP/534898/2008 Guideline on the Requirements for Quality Documentation Concerning Biological Investigational Medicinal Products in Clinical Trials. European Medicines Agency (EMA): London, UK, March 2012.
12 Parracho HMRT, et al. The Role of Regulated Clinical Trials in the Development of Bacteriophage Therapies. J. Mol. Gen. Med. 6, 2012: 279–286.
13 Pirnay JP, et al. Quality and Safety Requirements for Sustainable Phage Therapy. Products. Pharm. Res. 32, 2015: 2173–2179.
14 Nilsson AS. Phage Therapy: Constraints and Possibilities. Ups. J. Med. Sciences 119(2) 2014: 192–198; doi:10.3109/03009734.2014.902878.
15 Gill J, Hyman P. Phage Choice, Isolation, and Preparation for Phage Therapy. Curr. Pharm. Biotechnol. 11(1) 2010: 2–14.
16 Dennehy JJ. What Can Phages Tell Us About Host-Pathogen Coevolution? Int. J. Evol. Biol. 18 Nov. 2012: 396165; doi:10.1155/2012/396165.
17 Levin BR, Bull JJ. Population and Evolutionary Dynamics of Phage Therapy. Nat. Rev. Microbiol. 2(2) 2014: 166–173.
18 McCallin S, et al. Safety Analysis of a Russian Phage Cocktail: From Metagenomic Analysis to Oral Application in Healthy Human Subjects. Virology 443(2) 2013: 187– 196.
19 Skurnik M, Pajunen M, Kiljunen S. Biotechnological Challenges of Phage Therapy. Biotechnol. Lett. 29(7) 2007: 995–1003.
20 Parisien A, et al. Novel Alternatives to Antibiotics: Bacteriophages, Bacterial Cell Wall Hydrolases, and Antimicrobial Peptides. J. Appl. Microbiol. 104(1) 2008: 1–13.
21 No Action Today, No Cure Tomorrow: World Health Organization. Antimicrobial Resistance; www.who.int/world-health-day/2011/en; consulted 21 February 2016.
Bruno Speder is head of clinical regulatory affairs at SGS, [email protected]; www.sgs.com/cro.
You May Also Like






