The Green Imperative: Part One — Life-Cycle Assessment and Sustainability for Single-Use Technologies in the Biopharmaceutical IndustryThe Green Imperative: Part One — Life-Cycle Assessment and Sustainability for Single-Use Technologies in the Biopharmaceutical Industry
 Much has changed since large-scale single-use biomanufacturing equipment was introduced some 15 years ago. Since then, these materials have become accepted and established in production and downstream bioprocessing. Concerns about the environmental impact of single-use (SU) biomanufacturing equipment have become more prevalent as our environmental awareness has increased and related concerns have become more urgent (1). For example, many recommendations and even laws have emerged regarding plastic convenience packaging and products (2, 3). People have become more sophisticated in appreciating the distinctions and trade-offs in the pressures upon our air, water, and land. And we appreciate that, beyond passion and resolve, a science-based approach is required to design strategies for a circular economy. We must better understand the implications of industrial activities to reduce environmental stress caused by manufacturing, use, and disposal of single-use biomanufacturing equipment. Tools such as life cycle assessment (LCA) evaluate the absolute and relative effects of each manufacturing platform type on very specific categories of environmental stress (4). Industry must address concerns exposed by such analysis, and it needs to evaluate its activities and products to identify areas for further improvement.
Much has changed since large-scale single-use biomanufacturing equipment was introduced some 15 years ago. Since then, these materials have become accepted and established in production and downstream bioprocessing. Concerns about the environmental impact of single-use (SU) biomanufacturing equipment have become more prevalent as our environmental awareness has increased and related concerns have become more urgent (1). For example, many recommendations and even laws have emerged regarding plastic convenience packaging and products (2, 3). People have become more sophisticated in appreciating the distinctions and trade-offs in the pressures upon our air, water, and land. And we appreciate that, beyond passion and resolve, a science-based approach is required to design strategies for a circular economy. We must better understand the implications of industrial activities to reduce environmental stress caused by manufacturing, use, and disposal of single-use biomanufacturing equipment. Tools such as life cycle assessment (LCA) evaluate the absolute and relative effects of each manufacturing platform type on very specific categories of environmental stress (4). Industry must address concerns exposed by such analysis, and it needs to evaluate its activities and products to identify areas for further improvement.
Materials suppliers, manufacturers, and industry consortia alike are accepting that challenge. The biopharmaceutical and related industries are investing in “corporate social responsibility” through several means. One example is the Sustainability Committee established within the Bio-Process Systems Alliance (BPSA). The group is working to discover, collect, and distribute findings about sustainability and single-use technologies in bioprocessing. Here in the first of three articles, we introduce major themes arising in the study and implementation of single-use technology for a more sustainable manufacturing environment. The second article in this series will outline current thinking on how to design materials, platforms, and processes supporting the “rethink, reengineer, reduce, reuse, and recycle” paradigm of the circular economy for plastic and packaging principles (5) and is illustrated in Figure 1. Our final paper will illuminate current and future “end-of-life” handling methods and reprocessing technologies.
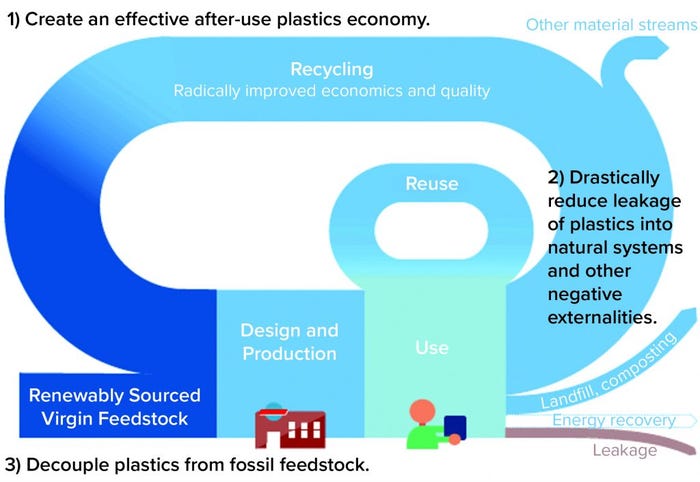
Figure 1: Illustration of circular economy concept for plastics (5); contrast closed-loop recycling (into the same or similar-quality application) with cascaded recycling (into other, lower-value plastics).
Life-Cycle Assessment
Environmental impacts or benefits in manufacturing often are considered in terms of a single consequence: e.g., energy efficiency, water consumption, carbon emissions, or solid waste. Such a perspective is efficient and easy, but it rarely captures the complexity and trade-offs that characterize the production of most materials and products. Furthermore, it is tempting to consider environmental consequences over a brief and arbitrary duration.
The LCA approach was developed over many years to enable examination of environmental impacts or benefits for products and manufacturing processes across a wide range of indicators (4). It is now an accepted technique for examining environmental impacts across the full life cycle of a given product: e.g., from raw-material extraction and refining through SU component production, use in biomanufacturing, and final end-of-life treatment. Assessing all steps in that life cycle provides insight into individual stresses and burden shifts from one step to another and enables understanding of both the cumulative environmental impacts and related trade-offs in each area, throughout the lifespan of both a facility and its manufactured products.
The first exhaustive LCA study of traditional and SU technologies to be published compared monoclonal antibody (MAb) manufacturing across many scales (6, 7). It evaluated the bioprocess from an n – 2 seed bioreactor through drug-substance purification, with the full life cycle for both traditional and SU biomanufacturing equipment from supply chain to waste disposal. The study began with materials and manufacturing of all process equipment and consumables supporting a 10-batch campaign. It concluded with end-of-life activities from treatment and disposal of consumables in SU as well as the disposal, reuse, or recycling of durable multiuse equipment used in traditional manufacturing.
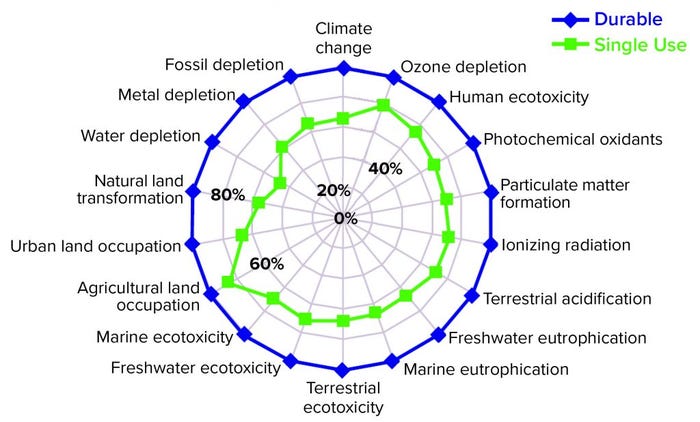
Figure 2: On average, SU facilities are more ecofriendly than traditional (durable) facilities in 18 distinct categories of environmental pressure (6, 7)
The results clearly pointed to the SU process train generally producing lower environmental impacts for each of 18 categories studied (Figure 2). The study also revealed otherwise hidden pockets of information that were not observable by looking at individual environmental burdens or stages of biomanufacturing. LCA studies have shown, surprisingly, that different options for postuse processing of SU material contribute an extremely small part of the total environmental impact of biomanufacturing (Figure 3).
A second detailed LCA study provided a deeper understanding of the potential impacts in SU process technologies (6). This study elucidated such important factors as the biopharmaceutical manufacturing facility’s geographic location. The two most influential variables revealed here were the environmental impact of power generation for the electrical grid and shipping of SU components to the biomanufacturing facility. Proximity to material end-of-life processing facilities is also important. In examining freshwater consumption, SU is always better than traditional biomanufacturing regardless of geography, electricity grid, or transport logistics. Similar to what was found for MAbs, adenovirus vaccine production in SU process technology also usually shows a lower environmental impact than the same process in durable equipment (7, 8). The “Generalizations” box summarizes significant conclusions from these published studies.
The studies were performed in a collaboration of SU consumable suppliers, biopharmaceutical manufacturers, and independent consultants. They examined both MAb and vaccine production, included different SU technologies, accounted for regional impacts, and examined several end-of-life treatment options. The authors considered effects upon such individual impact categories as climate change, energy and water usage, as well as combined categories grouping impacts according to each component’s stress upon ecosystem quality, human health, and/or natural resources.
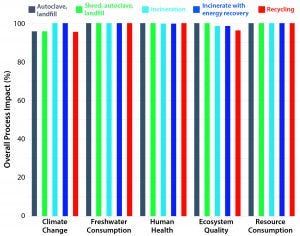
Figure 3: Comparative LCA-based environmental impact assessment of alternative end-of-life disposal options (8)
In examining hundreds of combinations of product, manufacturing technology, geography, and end-of-life options, those studies consistently show that SU technology usually presents a lower overall environmental impact than traditional multiuse technologies, largely because of reduced water consumption, decreased use of cleaning chemicals, and lower energy use. Factors included in the LCAs summarized here focused on areas such as raw materials, facilities, utilities, consumables, and labor that are affected by choosing SU equipment. Many assumptions and generalizations must be applied in such a study, and alterations of those assumptions do vary study conclusions to different degrees. Although such assumptions must be made to simplify both the calculations and presentation of the conclusions, it is nevertheless understood that such results generally represent the dynamics at hand. The studies revealed a number of relationships and correlations, both large and small.
Sustainability Goals and Corporate Responsibility
An estimated >300,000,000 tons of plastic waste is generated annually worldwide (5), with only 30,000 tons of that consumed by the biomanufacturing industry (9). Both of those numbers are growing, but the ratio appears to be constant. Even though biomanufacturing waste represents a very small fraction (0.01%) of the world’s total plastic waste, BPSA is concerned and its members are acting. We want to lead by example and do the best we can with the plastic that is in our direct control. SU-based biomanufacturing is often greener overall than traditional biomanufacturing, yet BPSA members are improving many parameters of their technologies, including sustainability, through initiatives supporting the superior design of materials, products, production systems, business activities, and postuse handling.
Corporate Responsibility Commitments: Many BPSA member companies have internal programs for reducing greenhouse gas production, water consumption or fouling, and energy consumption (10–15). SU product manufacturers are establishing zero-waste strategies in their operations and green criteria in their product development efforts. They are looking across the entire product life cycle and applying sustainability science and ecological engineering in designing packaging components, manufacturing solutions, and recycling programs. Whether it’s using renewable energy in manufacturing operations or reducing waste footprint in product applications, BPSA members are actively pursuing a range of creative measures as summarized in the “Creative Green Initiatives” box.
Sustainability By Design
Both suppliers and biomanufacturers are discovering that properly designed green initiatives also provide economic savings. For example, many programs that fall under the “Process Intensification” umbrella not only increase productivity by some unit of measure, but they also reduce environmental stress (e.g., by reducing material consumption). Using perfusion bioreactors to increase the density of cell culture and skip an n – x cycle not only saves time and expense, but it also serendipitously eliminates use of an entire cycle of SU materials. Engineering a clone to secrete more product in a given volume of medium not only increases productivity per dollar spent on media, but it also improves productivity per consumable piece.
Implementing prefabricated and modular components in facility design supports a more sustainable process in a number of ways. With reduced mass of construction materials, there is less to manufacture and ultimately to dispose of. Increased flexibility provides an increased likelihood of “future proofing” the results of construction. And installing suites with integral modular air handling — heating, ventilation, and air conditioning (HVAC) — ensures proper capacity and availability, and can obviate redundant systems. The second installment of this series will address designing and rethinking biomanufacturing products and processes to improve environmental impact.
Postuse Processing
Companies in all industries set waste-reduction goals that often are good for the environment, for productivity, and for profitability. Reducing waste — and potentially achieving zero waste — requires understanding scrap and why it is generated, segregating and measuring different waste streams, and then looking for the best home for each unavoidable stream. The key to achieving zero waste is finding an acceptable destination for hard-to-recycle items such as gloves, disposable garments, mixed plastic, and the complex multicomponent products used in biomanufacturing.
Some companies have been able to convert mixed plastics into such salable products as plastic lumber. Such material can be used in construction, decking, and landscaping. In partnership with postuse processors, BPSA members have codeveloped a plastic pallet made from plastic lumber. Beyond that, finding other products that can be developed using the processed mixed plastic waste continues to be a challenge. If end users are willing and prepared to disassemble/sort/segregate their plastics by type, then more options for upcycling would become available and a more circular economy could be created. Efforts to incorporate SU waste into broader plastic reclamation activities deserve special attention.
The disposal, recycling, and other postuse processing of SU components often form a major part of discussions and initiatives concerning SU technologies and the environment. In fact, it is often the first (or even only) aspect of such conversations, probably because of
the visibility of SU bags, tubing, and connectors
the reality that disposal or postuse processing of SU materials can be a significant fraction of a facility’s solid-waste management
the fact that that this aspect of the life cycle is within users’ direct control.
However, as Figure 3 illustrates, LCA studies have shown that the different options for postuse processing of SU material contribute a tiny part of the overall environmental impact of biomanufacturing. Nevertheless, because every contribution is important, BPSA members actively support the research and establishment of state-of-the art approaches to this matter. The final installment of this series will focus on current and future “end-of-life” handling methods including technologies for reprocessing.
Generalizations from Life-Cycle Assessment (LCA) Studies Comparing Traditional and Single-Use (SU) Systems
SU exhibits lower environmental impacts over aggregate life cycles. The greatest impact for both technologies is observed during the use stage. Water use (and consequences) is lower for SU across all life stages. End-of-life disposal environmental impacts are higher for SU systems. End-of-life impacts are negligible in the overall context of the entire life cycle. Supply-stage carbon/energy impact is higher for manufacturing and transport of SU. | Clean-in-place (CIP), steam-in-place (SIP), and water for injection (WFI) energy demands are the greatest burdens with stainless steel systems. Distance and mode of transport from component manufacturers drives the greatest burden for SU systems. No significant differences were observed among entity types, production scales or mixed modes. Facility geographical location greatly determines environmental impact through transport logistics and power grids. |
Healthcare and Single-Use
SU technologies supporting the manufacturing of biomedical products should be viewed differently than consumer-convenience goods such as plastic straws. Some SU technologies are critical components in the manufacture of life-saving and life-improving products. The healthcare industry has embraced these technologies because of the therapeutic safety and efficiency they provide. No one questions the infection control and patient support that SU syringes and surgical materials provide.
Similar benefits are provided to biopharmaceutical manufacturing and biomedical laboratory operations. Science-based studies clearly demonstrate that SU technologies can significantly reduce energy, water, and cleaning chemical consumption in biomanufacturing when compared with traditional process equipment. SU systems provide an overwhelming benefit by enabling safe, effective, and overall less-polluting systems for biomanufacturing (Figure 6).
SU material suppliers, integrators, and users of these technologies are committed to sustainability as good social and business practices — and this includes proper management of used materials. BPSA endorses the study of SU sustainability as well as implementation of new and better operational technologies that will limit further the impact of these materials on the environment. The social benefits of SU currently overwhelm the residual environmental risks, and BPSA will keep working to reduce those risks even further in the future. SU technology is a good choice now, and through these efforts it will become a better option in the future.
References
1 Whitford WG, Petrich MA, Flanagan WP. Chapter 13: Environmental Impacts of Single-Use Systems. Single-Use Technology in Biopharmaceutical Manufacture, Second Edition. Eibl R, Eibl D, Eds. John Wiley & Sons: Hoboken, NJ, 2019; doi:10.1002/9781119477891.ch13.
2 PE/11/2019/REV/1. Directive (EU) 2019/904 of the European Parliament and of the Council of 5 June 2019 on the Reduction of the Impact of Certain Plastic Products on the Environment. Off. J. Eur. Union 12 June 2019; https://eur-lex.europa.eu/eli/dir/2019/904/oj.
3 Environmental Project 1985: Life Cycle Assessment of Grocery Carrier Bags. Bisinella V, et al., Eds. Environmental Protection Agency: Copenhagen, Denmark, February 2018; https://www2.mst.dk/Udgiv/publications/2018/02/978-87-93614-73-4.pdf.
4 Balkau F, Fava J. Why Take A Life Cycle Approach? United Nations: Geneva, Switzerland, 2004; http://www.unep.fr/shared/publications/pdf/DTIx0585xPA-WhyLifeCycleEN.pdf.
5 MacArthur E, Waughray D, Stuchtey MR. The New Plastics Economy: Rethinking the Future of Plastics. World Economic Forum: Geneva, Switzerland, January 2016; http://www3.weforum.org/docs/WEF_The_New_Plastics_Economy.pdf.
6 Pietrzykowski M, et al. An Environmental Life Cycle Assessment Comparison of Single-Use and Conventional Process Technology for the Production of Monoclonal Antibodies. J. Clean. Prod. 41, 2013: 150–162; doi:10.1016/j.jclepro.2012.09.048.
7 An Environmental Life Cycle Assessment Comparison of Single-Use and Conventional Bioprocessing Technology. GE Healthcare Life Sciences: Uppsala, Sweden, 2013; https://www.gelifesciences.com/media/373d93eec4ae463b8c4bd7594802fa5d/19083-source/options/download.
8 Single-Use Technology and Sustainability: Quantifying the Environmental Impact in Biologic Manufacturing. GE Healthcare Life Sciences: Uppsala, Sweden, 2017; https://cdn.gelifesciences.com/dmm3bwsv3/AssetStream.aspx?mediaformatid=10061&destinationid=10016&assetid=16801.
9 Macdonald GJ. Single-Use Technology Has Biopharma Sizing Up the Recycling Bin. Gen. Eng. Biotechnol. News 1 August 2019.
10 Single Use and Sustainability. GE Healthcare Life Sciences: Uppsala, Sweden, 2020; https://www.gelifesciences.com/en/us/solutions/bioprocessing/knowledge-center/single-use-and-sustainability.
11 Environment. Thermo Fisher Scientific: Waltham, MA, 2020; https://www.thermofisher.com/us/en/home/about-us/corporate-social-responsibility/environment.html.
12 Environment: High Standard Environmental Protection. Sartorius AG: Göttingen, Germany, 2020; https://www.sartorius.com/en/company/corporate-responsibilty/environment.
13 Environmental Sustainability Overview. MSD Corporate Responsibility Report 2018–2019. Merck & Co.: Kenilworth, NJ, 2019; https://www.msdresponsibility.com/environmental-sustainability.
14 Our Values. Watson-Marlow Fluid Technology Group: Wilmington, MA, 2020; https://www.watson-marlow.com/us-en/about/our-values.
15 Sustainability. John Wood Group: Aberdeen, Scotland, 2020; https://www.woodplc.com/who-we-are/sustainability.
For Further Reading
ACS GCI Pharmaceutical Roundtable. American Chemical Society: Washington, DC, 2018; https://www.acs.org/content/acs/en/greenchemistry/industry-business/pharmaceutical.html.
Anastas P, Zimmerman J. Design Through the Twelve Principles of Green Engineering. Environ. Sci. Technol. 37(5) 2003: 94A–101A; https://pubs.acs.org/doi/10.1021/es032373g.
Brown A, et al. An Environmental Life Cycle Assessment Comparing Single-Use and Conventional Process Technology. BioPharm Int. 17, November 2011: S30–S38; http://www.biopharminternational.com/environmental-life-cycle-assessment-comparing-single-use-and-conventional-process-technology.
Budzinski K, et al. Toward Sustainable Engineering Practices in Biologics Manufacturing. BioProcess Int. eBook, 18 December 2015: https://bioprocessintl.com/manufacturing/single-use/toward-sustainable-engineering-practices-in-biologics-manufacturing.
Caine B. The Impact of Single-Use Technologies. Bio-Process Systems Alliance: Arlington, VA, 2009; http://www.bpsalliance.org/wp-content/uploads/2014/06/2009-SUS-Survey.pdf.
Disposals Subcommittee of the Bio-Process Systems Alliance. Guide to Disposal of Single-Use Bioprocess Systems. BPSA: Arlington, VA, 2014; http://www.bpsalliance.org/wp-content/uploads/2014/06/BPSA-Disposal-Article-BPI-1107.pdf.
Flanagan W, et al. An Environmental Life Cycle Assessment of Single-Use and Conventional Process Technology: Comprehensive Environmental Impacts. BioPharm Int. 27 (3) 2014: 40–46; http://www.biopharminternational.com/environmental-lifecycle-assessment-single-use-and-conventional-process-technology-comprehensive-envi.
Flanagan W. Single-Use and Sustainability: GE Quantifies the Environmental Impact. BioProcess Online 2016; https://www.bioprocessonline.com/doc/single-use-technology-and-sustainability-quantifying-the-environmental-impact-0001.
Hearn MT. Recent Progress Toward More Sustainable Biomanufacturing. Prep. Chromatogr. Separat. Proteins 2017: 537–582.
Idris A, et al. Systematic Methodology for Evaluating Environmental Impact of a Biopharmaceutical Production: A MAbs Case Study. The World Research and Innovation Convention on Engineering and Technology (WRICET) 2012; https://www.researchgate.net/publication/250310518_systematic_methodology_for_evaluating_environmental_impact_of_a_biopharmaceutical_production_a_mabs_case_study.
IPCC. Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press: Cambridge, UK, 2007.
ISO 14040: Environmental Management – Life Cycle Assessment – Principles and Framework. International Organization for Standardization: Geneva, Switzerland, 2006.
ISO 14044: Environmental Management – Life Cycle Assessment – Requirements and Guidelines. International Organization for Standardization: Geneva, Switzerland, 2006.
Junker B. Minimizing the Environmental Footprint of Bioprocesses, Part 1: Introduction and Evaluation of Solid-Waste Disposal. BioProcess Int. 8(8) 2010: 62–71; https://bioprocessintl.com/manufacturing/supply-chain/minimizing-the-environmental-footprint-of-bioprocesses-302914.
Junker B. Minimizing the Environmental Footprint of Bioprocesses, Part 2: Evaluation of Wastewater, Electricity, and Air Emissions. BioProcess Int. 8(9) 2010: 36–46; https://bioprocessintl.com/manufacturing/facility-design-engineering/minimizing-the-environmental-footprint-of-bioprocesses-303905.
Lopes AG. Single-Use in the Biopharmaceutical Industry: A Review of Current Technology Impact, Challenges and Limitations. Food Bioprod. Proc. 93, 2015:98–114.
Mahajan E, et al. One Resin, Multiple Products: A Green Approach to Purification. Dev. Biotechnol. Bioproc. 2013: 87–111; doi:10.1021/bk-2013-1125.ch006.
Mata TM, et al. LCA Tool for Sustainability Evaluations in the Pharmaceutical Industry. Chem. Eng. Trans. 26, 2012: doi:10.3303/CET1226044.
Mauter M. Environmental Life-Cycle Assessment of Disposable Bioreactors. BioProcess Int. 7(4) 2009: 18–28; https://bioprocessintl.com/manufacturing/supply-chain/environmental-life-cycle-assessment-of-disposable-bioreactors-184138.
Noce A. 25 Years of Green Chemistry. LinkedIn 14 July 2017; https://www.linkedin.com/pulse/25-years-green-chemistry-anthony-noce-acsf/?trk=mp-readercard.
Pietrzykowski M, et al. An Environmental Life Cycle Assessment Comparing Single-Use and Conventional Process Technology. BioPharm Int. 24 (11) 2011 : S30–S38; http://www.biopharminternational.com/environmental-life-cycle-assessment-comparing-single-use-and-conventional-process-technology.
Pietrzykowski M, et al. An Environmental Life Cycle Assessment Comparison of Single-Use and Conventional Process Technology for the Production of Monoclonal Antibodies. J. Clean. Prod. 41, February 2013: 150–162; doi:10.1016/j.jclepro.2012.09.048.
Pora H, Rawlings B. Managing Solid Waste from Single-Use Systems in Biopharmaceutical Manufacturing. BioProcess Int. 7(1) 2009: 18–25; https://bioprocessintl.com/manufacturing/supply-chain/managing-solid-waste-from-singleuse-systems-in-biopharmaceutical-manufacturing-183494.
Ramasamy SV, Titchener-Hooker NJ, Lettieri P. Life Cycle Assessment As a Tool to Support Decision Making in the Biopharmaceutical Industry: Considerations and Challenges. Food Bioprod. Process. 94, 2015: 297–305; doi:10.1016/j.fbp.2014.03.009.
Rawlings B, Pora H. A Prescriptive Approach to Management of Solid Waste from Single-Use Systems. BioProcess Int. 7(3) 2009: S40–S47; https://bioprocessintl.com/manufacturing/supply-chain/a-
prescriptive-approach-tomanagement-of-solid-waste-from-single-use-systems-183658.
Rawlings B, Pora H. Environmental Impact of Single-Use and Reusable Bioprocess Systems. BioProcess Int. 7(2) 2009: 18–25; https://bioprocessintl.com/manufacturing/supply-chain/environmental-impact-of-single-use-and-reusable-bioprocess-systems-183572.
Reid WV, et al. Ecosystems and Human Well-Being: A Report of the Millennium Ecosystem Assessment. World Health Organization: Geneva, Switzerland, 2005; http://apps.who.int/iris/bitstream/handle/10665/43354/9241563095.pdf;jsessionid=9F90A4BDE3F3F1B0BDA88C7EC6330820?sequence=1.
Scott C. Sustainability in Bioprocessing: Not Just an Afterthought. BioProcess Int. 9(10) 2011: 25–36; https://bioprocessintl.com/manufacturing/monoclonalantibodies/sustainability-in-bioprocessing-323438.
Shukla AA, Gottschalk U. Single-Use Disposable Technologies for Biopharmaceutical Manufacturing. Trends Biotechnol. 31(3) 2013: 147–154; doi:10.1016/j.tibtech.2012.10.004.
Sinclair A, et al. The Environmental Impact of Disposable Technologies. BioPharm Int. 21(11) 2008: S4–S15; https://biopharmservices.com/wp-content/uploads/2014/04/EnvironmentImpactDisposables.pdf.
Toke LK, Gupta RC, Dandekar M. Green Supply Chain Management: Critical Research and Practices. Proceedings of the 2010 International Conference on Industrial Engineering and Operations Management (Dhaka, Bangladesh, 9–10 January 2010). IEOM International: Canton, MI, 2010; https://www.researchgate.net/publication/
266464477_Green_Supply_Chain_Management_Critical_Research_and_Practices.
Wells B, et al. Guide to Disposal of Single-Use Bioprocess Systems. BioProcess Int. 6(5) 2008: 24–27; https://bioprocessintl.com/manufacturing/supply-chain/guide-to-disposal-of-single-use-bioprocess-systems-183977.
Whitford WG, Scott C. Single-Use and Sustainability. BioProcess Int. 12(4) 2014: S12–S17; https://bioprocessintl.com/2014/single-use-andsustainability-351059.
World Energy Outlook-2017. International Energy Agency: Paris, France, 2017; http://www.iea.org/weo2017.
Magali Barbaroux is head of corporate research at Sartorius Stedim Biotech; Brian Horowski is director of process technologies at Wood Life Sciences; Sade Mokuolu is global product compliance manager at Watson-Marlow Fluid Technology Group; and Mark Petrich is both director of single-use engineering at Merck and first vice-chair of the Bio-Process Systems Alliance (BPSA). Corresponding author and BPI editorial advisor William Whitford is bioprocess strategic solutions leader for Cytiva in Logan, UT; [email protected]. Bill Flanagan is cofounder and director of Aspire Sustainability in Breckenridge, CO; https://aspiresustainability.com.
This paper represents conclusions of the BPSA sustainability subcommittee and not necessarily specific viewpoints of the companies represented by its members.
You May Also Like






