Untapped Potential of Tissue Engineering: The Three Obstacles Holding It BackUntapped Potential of Tissue Engineering: The Three Obstacles Holding It Back
Regenerative medicine is the interdisciplinary field comprising tissue engineering, cell therapy, and gene therapy. These biopharmaceutical modalities, also referred to as advanced therapies, are growing rapidly, characterized by groundbreaking therapeutic advances that have the potential to change how healthcare providers deliver care. As Figure 1 shows, cell and gene therapies have gained traction over the past decade, as evidenced by large increases in investment and the number of marketed products. By contrast, tissue engineering investment and product commercialization has lagged (1). More than 1,000 companies are developing cell and gene therapies, but only about 130 of them are exploring tissue-engineered medical products (TEMPs) as a viable source of regenerative therapeutics.
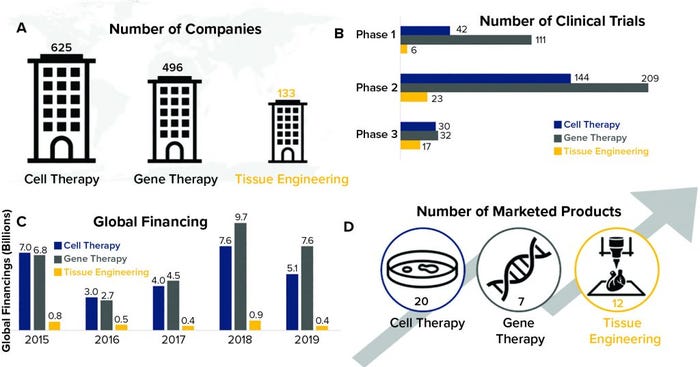
Figure 1: Temporal comparison of cell therapy, gene therapy, and tissue engineering (data from the Alliance for Regenerative Medicine, July 2020)
Based on our analysis, we believe that the delayed maturation of tissue-engineered drug products is caused primarily by the greater relative complexity of TEMPs compared with cell and gene therapies. Complexity in this sense refers to both the structural composition of the drug product and the associated manufacturing process. That elevated complexity has manifested itself in three interrelated difficulties for the tissue-engineering field: complex manufacturing, ambiguous regulation, and insufficient reimbursement.
Complex Manufacturing
Manufacturing is relatively more complex for TEMPs than it is for cell and gene therapies because of the required raw materials, uncertainties of quality control, and difficulties scaling production capabilities. Those issues have deterred some companies from developing TEMPs. Some companies that have faced such problems have failed to recoup the cost of their investments. For example, Smith & Nephew faced difficulties in scaling capabilities to manufacture Dermagraft tissue-engineered skin substitute for treating diabetic foot ulcers. Despite achieving approval from the US Food and Drug Administration (FDA) and showing clinical benefits, Dermagraft has failed repeatedly to turn a profit — in part because of its complicated manufacturing requirements.
Compared with cell and gene therapies, tissue-engineered products require additional raw materials in the form of cells, scaffolds, and signaling molecules, which are not always easily combined based on a predetermined recipe. The quantity and type of raw materials used in TEMP production have significant effects on end products.
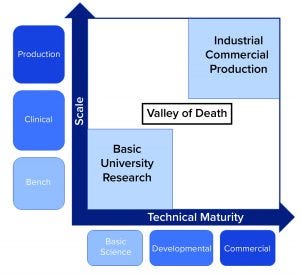
Figure 2: “Valley of death” for TEMPs progressing toward commercialization caused by decreased investor confidence (source: CRA analysis)
Biomanufacturers must assess and balance carefully each of the required ingredients and determine how they can influence the validity of a drug product. Questions drug developers should ask include “Which signaling molecules induce appropriate cellular differentiation? Does the scaffold provide a suitable environment for cellular growth and vascularization? Does the end product possess the appropriate structural and mechanical properties?” For most biomanufacturers, the answers to such questions largely remain uncertain.
Other difficulties include ensuring quality control, consistency, and reproducibility of TEMPs. With limited options for in-line, nondestructive testing of critical quality attributes (CQAs), accurately assessing TEMPs before manufacturing processes are complete often is infeasible. In general, TEMPs are manufactured by manually combining cells from a patient’s body with scaffold biomaterials. That presents considerable challenges when preparing to scale up production. Such a manual production approach often is used when TEMP manufacturers establish proof of concept. But as their products progress to larger scales, those companies will need to invest in infrastructures and advanced technologies to scale up production rapidly and ensure end-product quality and reproducibility.
Ambiguous Regulations
A lack of clear regulatory pathways for TEMPs also limits their advancement. Existing regulatory pathways do not address the added complexity of TEMPs appropriately. TEMP manufacturers should collaborate with the FDA and other regulatory agencies as early as possible during clinical development to align on regulatory requirements for approval. Both parties will need to agree on the amount of clinical data needed, which often can be limited (2).
TEMP manufacturers also need to determine the appropriate assessment of manufacturing processes for their products. Few options might be available for in-line, nondestructive testing of CQAs, which could make demonstrating production quality and consistency difficult. If manufacturers readily could demonstrate appropriate levels of quality and consistency, then regulatory agencies more easily could differentiate one product from another and make systematic decisions on how best to regulate TEMPs (3). Some regulatory agencies have made considerable progress toward streamlining regulations of TEMPs in recent years, but regulators and biomanufacturers should continue to engage frequently with each other throughout clinical development to align on the most effective solutions moving forward.
Insufficient Reimbursement
Universally accepted standards are lacking in accurate valuation and reimbursement of curative cell and gene therapies and TEMPs. Curative therapies can generate long-term benefits with a single or limited number of applied doses, which are valued based on their clinical benefit, effect on patients’ quality of life, overall healthcare system costs, and added societal value. Typically, because of their high upfront costs, curative therapies have been scrutinized extensively, raising questions about how they should be funded.
Healthcare systems around the world, including nationalized single-payer healthcare systems such as those in Europe and segmented multipayer healthcare systems such as that in the United States, are working to evolve their policies and reimbursement strategies to address the many unique and complex factors associated with curative cell and gene therapies. Industry stakeholders are collaborating and considering alternative reimbursement models (e.g., cost-sharing, value-based contracts) and/or financing models (e.g., reinsurance, installment payments, and consumer health loans) to better support those therapies. New and emerging reimbursement models being developed for curative cell and gene therapies could be applied to TEMPs in the years ahead to help healthcare systems effectively value and reimburse expensive and complex products (4).
Overcoming Obstacles
Difficulties presented by complex manufacturing processes and uncertainties regarding regulatory pathways and reimbursement protocols have led to decreased investor and clinical confidence in TEMPs. Most investors in the biopharmaceutical industry understand the uncertainties and risks involved with investigational therapies. But the added complexity of TEMPs has brought about a lack of investment in that sector. Associated risks seem to outweigh the promise of innovative TEMPs, creating a bottleneck of novel TEMPs in clinical development stages and reducing the likelihood of their commercialization.
But as development and investment in cell and gene therapies continues to advance, lessons learned from their developers, manufacturers, and other stakeholders might be applied to the tissue-engineering sector to help accelerate development of TEMPs. Stakeholder groups such as payers, regulators, and manufacturers must consider collaborating frequently and early in the clinical development process of TEMPs to align on regulatory and manufacturing protocols. Manufacturers also might need specialized expertise on optimal production strategies to ensure reliable, reproducible, and consistent manufacturing processes that are rapidly scalable for commercialization (5).
References
1 Ghaemi R, Siang LC, Yadav VG. Improving the Rate of Translation of Tissue Engineering Products. Adv. Healthc. Mat. 8(19) 2019: 1900538; https://doi.org/10.1002/adhm.201900538.
2 Gardner J, Webster A. The Social Management of Biomedical Novelty: Facilitating Translation in Regenerative Medicine. Social Sci. Med. 156, 2016: 90–97; https://doi.org/10.1016/j.socscimed.2016.03.025.
3 Kim YS, et al. An Overview of the Tissue Engineering Market in the USA from 2011 to 2018. Tissue Eng. Part A 25(1–2) 2018: 1–8; https://doi.org/10.1089/ten.tea.2018.0138.
4 Mount N, et al. Cell-Based Therapy Technology Classifications and Translational Challenges. Philos. Trans. R. Soc. Lond. B Biol. Sci. 307(1680) 2015: 20150017; https://doi.org/10.1098/rstb.2015.0017.
5 O’Donnell BT, et al. Beyond the Present Constraints That Prevent a Wide Spread of Tissue Engineering and Regenerative Medicine Approaches. Front. Bioeng. Biotechnol. 7, 2019: 95; https://doi.org/10.3389/fbioe.2019.00095.
Corresponding author Lev Gerlovin is a vice president, Andrew Thomson is a consulting associate, and Jack Vailas is an associate within the Life Sciences Practice at Charles River Associates (CRA), 200 Clarendon Street, Boston, MA 02116; [email protected].
The views expressed here are those of the authors and not those of their employer or other organizations with which they are affiliated.
You May Also Like






