21st Century Vaccine Manufacturing21st Century Vaccine Manufacturing
Establishment of standard production platforms can help vaccine development move a step closer to the commercial, technical, and regulatory benefits increasingly enjoyed by developers of monoclonal antibody (MAb) products. Three recent advances especially will assist vaccine manufacturing development: rapid analytical methods to support evaluation of process design and provide in-process control; and the establishment of supply chains and vendors across Asia for bioprocessing equipment and consumables that meet the highest international standards. Whereas some workers in the field may consider the goal of achieving mass vaccination through a standard production platform impractical, there will be tremendous benefits if the technical and commercial problems can be resolved.
Manufacturing Design and Development Challenges
MAbs are now firmly established as a mainstream class of biopharmaceuticals with a reasonably generic platform manufacturing design (1,2,3). There is an expanding range of contract manufacturing organizations (CMOs) offering MAb production that allows outsourcing strategies to be adopted by many product-sponsor companies.
From a technical/regulatory perspective, there are significant opportunities to accelerate times to the clinic and the market as well as optimize process performance and economics during MAb manufacturing development.
Comparability protocols are used to define the characterization criteria for both process and product, assisting in scale-up and process optimization and thus minimizing the risk of invalid preclinical and clinical data sets. This has been brought about in part by advances in analytical technology and through expertise gained by working on the large numbers of MAb products either under development or approved.

Figure 1:
(WWW.NUNCBRAND.COM)
Such experience is not merely confined to drug developers, but also applies to regulatory authorities. For example, the introduction of a new master cell bank reengineered to be more productive partway through clinical development would have been a change most regulatory personnel would recoil from considering back in the 1980s. Now, two decades’ experience with such approaches are used by some biopharmaceutical companies as a means to expedite first-in-man studies, and they can be accepted by regulatory authorities when supported with appropriate data.
In short, because of generic manufacturing and analytical platforms (combined with a well-informed regulatory position), MAbs have become in many ways similar to small-molecule pharmaceuticals (as summarized in the “MAb Evolution” box) and thus MAb developers are enjoying the benefits this brings.
Challenges During Vaccine Manufacture
By contrast, vaccine manufacturing design offers significantly different challenges and opportunities because vaccines remain firmly anchored to the process = product paradigm. Their complexity from a manufacturing design standpoint reflects two factors: product biochemical diversity and production platform variety. The interaction of those two factors — in combination with regulatory risk/benefit evaluation for healthy subjects rather than patients presenting with a specific disease for treatment — significantly increases the manufacturing design challenges for vaccines.
The complex range of vaccine products ranges from live organisms (e.g., attenuated Salmonella typhi) to inert proteins (e.g., virus-like particles used to protect against hepatitis B infection). Clearly such product diversity profoundly affects facility design, process validation, and control. For example, with a live microbial vaccine, how do you assure sterility when terminal sterilization using 0.2-µm filtration is infeasible? Is it possible to change the bacterial strain during clinical development? (The answer to that question is clearly not without significant rework because in this situation the process = product paradigm is an absolute.) These diverse product types in turn require diverse production technologies, particularly for upstream production of crude antigens: e.g., bacterial and yeast fermentations, cell cultures based on a range of cell substrates, and baculovirus-based insect cell transient expression. Figure1 illustrates the impact of product and process complexity for vaccine development.

Figure 1: ()
MAb Evolution
Monoclonal antibodies are becoming like small-molecule drugs in some ways:
Commercial Trends: MAbs are now a mainstream pharmaceutical product class, with many biotechnology companies working with product portfolios made up of small molecules and MAbs.
Manufacturing Trends: The largely generic manufacturing design for MAb production and processing is now easily outsourced. An expanding international range of commercial facilities is employing the available experienced scientists and engineers.
Regulatory Trends: Comparability protocols support the flexibility of scale-up. Under CDER (rather than CBER) oversight, process analytical technologies and quality by design initiatives are being applied.
One general benefit of vaccines, however, is that their required clinical doses are typically much smaller than those for MAbs. With doses in the microgram range, vaccine production volumes for market can be provided at production scales of 100–2,500 L rather than the 5,000–20,000 L more typically associated with commercial MAb production. The ability to manufacture commercial supplies of some vaccines at relatively small production volumes introduces even greater variability in vaccine production technology through the use of flasks, roller bottles, and other production systems more typically used only in preclinical MAb development. Manufacturing information within EU market authorization documents verify both the comparatively low volumes and variety of production technologies used: Hexavac liquid hexavalent vaccine 600–800 L; Varivax varicella virus vaccine, roller bottles; Rotarix rotavirus vaccine, Nunclon Cell Factory systems (www.nuncbrand. com); Optaflu influenza vaccine 2,500 L; HbVaxpro hepatitis B vaccine 2,100 L, and Dukoral cholera vaccine 500 L (4).
Similarly, whereas the design of purification schemes for MAbs has typically become almost generic in nature, with predominant use of protein A chromatography for primary capture followed by two additional chromatography unit operations for polishing with filtration interspersed among them, the variables of vaccine type, production system, and scale have precluded establishment of a single dominant “plug-and-play” platform approach to purification. Bioprocess development for vaccines remains technically challenging, with expertise often located within a few companies that is thus not readily accessible.
The number of CMOs with vaccine expertise is much smaller than those focused on MAbs, further compounding the challenge of vaccine development. The difficulty sometimes associated with identifying a suitably qualified and equipped vaccine CMO has contributed to the decision by many biotechnology companies developing vaccines to establish their own CGMP production facilities despite the commercial risks and use of capital that might otherwise be used to expand product development portfolios.
Trends in MAb development and regulation such as the FDA Center for Drug Evaluation and Research’s (CDER’s) stance on comparability protocols and adoption of quality by design (QbD) signal the way forward for simplifying and speeding scale-up postapproval for recombinant protein-based therapeutics. Vaccines, by contrast, remain firmly anchored to the process = product paradigm. The implications are clear: When developing vaccines, it is necessary to begin with the end in mind and fix the production strain, process, and formulation as early as possible. Otherwise, even relatively minor changes during product development risk impairing both preclinical and clinical data sets.
For example, generating a scientific argument regarding the essential similarity of a clinical trial vaccine generated by earlier process configurations is likely to be hindered by the greater challenge in fully characterizing complex purified antigens or vaccine presentation. This gives rise to potential time and cost implications of repeating preclinical studies before phase 2 clinical studies can commence. The same issues pose implications for clinical data. Consider the impact, at a pre-BLA meeting with CBER representatives, of finding that the regulator has concerns over the size of a safety database of individuals exposed, which is a fundamental criterion for a vaccine product. If the regulator then considers materials in early stage trials to be unrepresentative of the late-phase products, they will restrict the database to phase 3 studies with the final definitive product and process. As a consequence, earlier clinical studies end up providing only support rather than pivotal data. Unfortunately, this is not a hypothetical situation, but rather comes from our own direct experience in which additional clinical trials were required, delaying the submission of a license application by over a year.
With all these challenges in mind, how can vaccine development and commercial production move a step closer to the benefits increasingly enjoyed by developers of MAb products? We consider three advances to be offering hope for the future: opportunities to establish common production platforms based on standard unit operations (bioreactor culture and chromatographic purification); and the adoption of rapid analytical methodologies to create the kind of data-rich environment enjoyed by MAb process scientists; and establishment of robust supply chains of bioprocess equipment and consumables across Asia.
Toward Standard Production Platforms
The barriers are too great to envisage redesigning existing licensed vaccines to fit a common platform. But the vaccine industry as a whole would benefit by establishing template processes for different vaccines as they progress from research to development. Based on a platform vaccine production approach, it would seem reasonable to propose such upstream and downstream unit activities as scalable bioreactor suspension cell culture and purification processes that are transferable among different vaccines types.
Bioreactor Suspension Cell Culture: Despite its potential benefits, manufacturing all vaccines in bioreactors with suspension cell culture represents the greatest challenge for the diversity of product types. General starting factors critical to a successful strategy for bioreactor suspension cell culture will include the following:
Establishing a small range of well-characterized, expression-permissive cell lines/strains suspension-adapted in serum-free culture (when appropriate) for use as cell substrates in vaccine production
Major vendors supplying comparative bioreactor systems (both stainless steel and disposable) that can be installed worldwide and are designed to facilitate rapid, small-scale, platform development while reducing the risk of process variance when transferred to larger production capabilities
Training on operation and data analysis of bioreactor cultures.
None of those factors are insurmountable in time, and great strides have been taken toward realizing the ideals. The movement away from egg-based influenza vaccine manufacture to the use of cell-culture–based production demonstrate that this concept is possible. In the case of cell-based flu vaccine production, some manufacturers have used adherent culture on microcarriers rather than suspension-adapted cells. This fails our criteria because microcarriers impose additional complexity, such as variable levels of colonization per carrier and cleaning validation that’s ideally best avoided.
In addition, recent focus on media formulations has led to advances in the development of suspension HEK293 cultures for production of adenovirus serotype-2 and -5 (Ad2 and Ad5) vaccines. Development of chemically defined and typically serum-free formulations has not only served to increased product titers and patient safety (through the absence of bovine serum additions), but also significantly expedited first-in-man studies through the ability to successfully scale the developed process (5). However, the production scale required to support later-stage clinical studies — and certainly market supply — would suggest that further upstream developmental improvements are required (6).
By contrast, the challenges for recombinant microbial vaccine product are not typically focused toward the mode of fermentation but rather the fundamental biology of vaccine expression. Advances in expression technologies have provided benefits from increases in product titer (based around vector design, positional integration, and episomal copy number events) and in some cases preferential antigen presentation with associated increases in vaccine efficacy (7).
Establishment of robust disposable bioreactor systems may facilitate further use of suspension culture for vaccine manufacture. To support all vaccine types, however, would require performance comparable to a stainless steel system for both mammalian and microbial cultures in growth, cell physiology, and productivity. Experience with current systems suggests that they do not as yet universally attain this ideal. Mammalian-cell bioreactors based on rocking platforms can create shear damage on scale-up, altering the impurity profile of a purified antigen. Alternatively, those based on emulating a stirred tank may not be able to achieve stirrer speeds or mass transfers suitable for microbial cultures. However, a number of vendors are actively developing in this area, and such systems should become available in the near future.
As for bioreactor supply, it is notable from our experience during technical visits to biomanufacturing facilities in Asia (including Japan, South Korea, Taiwan, and India), that although major US/European vendors are used for purification equipment, it is more common for bioreactors to be sourced locally.
Training in bioreactor operation within the vaccine industry can take advantage of the expansion of the MAb sector as a source of experienced scientists and engineers. Moreover, development scientists gain experience in the three-way interplay of regulatory science and quality assurance with process science, which is core to successful product development, by practical application within organizations developing biopharmaceuticals.
How much more can this knowledge expand if a common production platform is adopted? Current vaccine production systems include not only bioreactors, but cell flask systems, roller bottles, and so on operated both manually and by robots. In a research setting, those are simply a variation on the theme of culturing cells cheaply, but in a CGMP environment the variety of different systems creates additional challenges relating in particular to manufacturing different product types. For example, different modes of failure need to be controlled, multiple vendors need to be audited, operator training must be documented, and so on. Increased use of small-scale (disposable) stirred-tank fermentors will increase market volumes and drive down prices, making bioreactors more attractive for research laboratories to produce antigens for preclinical research purposes.
Transferable Purification Processes: Although it has obvious benefits, a standardized platform for vaccine purification has been difficult to achieve because of the diversity of product types and the typically complex nature of the biomolecules and organisms concerned. However, recent technological advances are enabling vaccine purification schemes that are not only efficient, scalable, and regulatory compliant, but also transferable among different product types.
One example comes in the production of viruses and virus-like particles (VLPs). Traditionally, the primary purification step has been density-gradient centrifugation, which continues to be used in clinical and commercial manufacture of vaccines for such infections as influenza, hepatitis B, and Japanese encephalitis. However the scale-up of a density-gradient purification step is often restricted to installing multiple units, and such systems are not as readily installed or replaced as other purification systems can be. So there has been a move toward chromatography-based techniques for purification of these products.
Column chromatography has an established history in the production of virus vaccines and VLPs but mainly has been used in final polishing steps and for the removal of specific contaminants such as DNA and liposaccharides. Application of conventional packed bed-chromatography as a primary capture step is limited, however, by the large diameter of virus/VLP molecules sterically restricting diffusion into bead pores, in which the majority of the active binding sites are located. Thus, binding capacities are much reduced, resulting in lower yields and making larger columns necessary.
Binding capacities may be improved with chromatography using membranes or monolithic columns with wider pore/channel diameters than those in resin beads. As such, a greater proportion of active chemistry is available to target molecules, greatly increasing binding capacity. Another advantage associated with membrane and monolith operation is high volumetric flow rates, which allow for smaller columns and shorter cycle times. All these benefits are making chromatographic separations an increasingly attractive and economically viable option for purification of very large molecules such as vaccines (8,9).
Not all vaccines can be purified using chromatographic approaches. For example, those based on whole-cell preparations will be too large to pass through even the relatively wide channels of membranes and monolithic supports. Further advances in ultrafiltration technology, including reduction of nonspecific adsorption, will facilitate the development of scalable purification processes for such products.
Advances in purification technology for macromolecules and whole cells should use the larger molecular weight of vaccines advantageously. It is relatively easy to separate away smaller contaminating molecules, such as host-cell proteins and nucleic acids, lipids, and sugars. This suggests that platform systems, although requiring optimization according to vaccine type, could contain many of the same unit operations. We have adopted this philosophy when producing a range of VLPs and viral vectors. Learning from our experience in producing VLPs, we have developed processes for the manufacture of gene-therapy viral vectors (Figure 2). Certain stages, such as the method for cell lysis, obviously will be unique for each product. However, other unit operations will be very similar and in some cases identical. Our strategy is to further explore which purification steps can be transferred among products, with the aim of consolidating toward standardized platforms wherever possible.
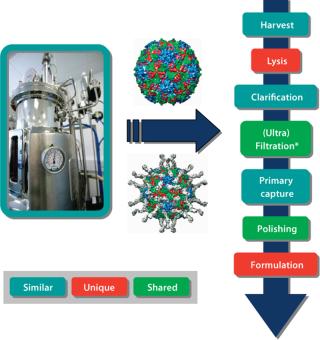
Figure 2: ()
Adoption of Rapid Analytical Methodology: Analysis of vaccines, particularly whole inactivated microorganisms or live viruses, has tended to persist with methods used since the 1970s (e.g., dry cell weight, DCW, in the case of inactivated microorganisms and plaque forming units, PFU, with viruses). Although such methods are still valuable and likely to be retained as in-process control or lot-release tests, more rapid or sensitive analytical methods are becoming available that can assist in vaccine process development. Successful development of biopharmaceuticals depends on high-quality analysis to interpret process design and development studies. Of particular benefit are analytical methods that can be applied not only to final purified samples, but also throughout production, because that allows a process to be more meaningfully evaluated. Analysis of large complex biomolecules such as vaccines, however, has long presented a challenge for process development and quality control.
The simple fact that many multicomponent vaccines are so large relative to recombinant biopharmaceuticals has rendered many traditional bioanalytical methods obsolete. By comparison, spectroscopic or chromatographic methods may be considered more suitable for analysis of larger biomolecules. In our laboratories, for example, we have evaluated various methods to provide rapid, high-throughput analysis of in-process and purified viral product samples to support the successful and rapid development of a high-titer, platform viral production process. For example, we have applied novel monolithic HPLC columns (such as Agilent’s Bio-Monolith QA brand, www.chem. agilent.com) to monitor purification of type-5 adenovirus particles Once developed, the method has provided rapid analysis (<10 minutes) of samples throughout our entire process, enabling well-informed decisions to be made during process development (10).
Toward Widespread Equipment Supply
Modern MAb manufacturing is currently being transferred to Asia by major corporations either to supply local markets or to leverage labor cost and tax advantages with the intention of exporting Asian-manufactured products to western markets. Development and establishment of MAb commercial manufacturing in Asia combined with efforts by Asian manufacturers to enter the biosimilar arena is creating a new market for major global vendors of bioprocess equipment and raw materials. Asian biomanufacturing corporations wishing to operate to western standards or — in the case of biosimilars, emulate biopharmaceutical innovators — currently have to import almost all their process equipment, media components, and disposable equipment.
At a recent conference in Mumbai, one of India’s largest corporations indicated that the cost for its new biopharmaceutical facility was around 60–70% more than US costs for the same facility would have been because most equipment had to be imported. As this market grows, a tipping point will be reached, and it will make more economic sense for suppliers to manufacture and supply equipment and consumables (e.g., disposable bioreactors and media), within Asia to the highest international standards. Such development would benefit codevelopment of vaccines and commercial supply to a global population no matter whether the site of manufacture is Mumbai, Munich, Melbourne, Manchester, or Massachusetts.
Standard Equipment Designs: Establishment of a common production template for MAb manufacture has assisted regulators and manufacturers and ultimately helped patients gain access to products that will advance health care in the 21st century. However, as described herein, there are fundamental differences in the manufacture of vaccines and MAbs even when both are derived from cell culture: the much wider range of vaccine types, with an associated broad range of necessary production technologies and a need for relatively small production scales to supply the market. Vaccines are usually complex, as well, and as such difficult to fully characterize.
The range of product types may be broad, but the range of production technologies can and should be actively reduced. Equipment vendors have a part to play in this progress, and the industry can take advantage of evolving manufacturing “out-of-a-box” approaches that combine single-use technologies for all unit operations. Disposables lend themselves particularly well to vaccine manufacture for three reasons: vaccine production tends to run in campaigns to produce bulk purified product (rather than continuous production), facilities need to be flexible and capable of supporting multiple products for commercial supply (cleaning validation for multiproduct facilities is a particularly acute problem with many classes of vaccine), and relatively low production volumes are required to generate prophylactic vaccines compared with therapeutic antibodies.
Standard equipment designs will accelerate the rate of global supply and increase access to prophylactic (and, in time, therapeutic) vaccination. Advocating the ultimate goal of a series of predefined generic platform process backbones as templates for production of different vaccine classes may seem counter to innovations in process technology. However, the general trend toward increased product complexity ensures that manufacturing development will retain plenty of challenges to confront development scientists in the 21st century. Some may consider the challenges insurmountable, but the same was probably said during the early development of laboratory automation and robotics for cell culture, which are now used routinely by vaccine manufacturers in Asia, Europe, and North America.
REFERENCES
1.) Slaff, G.Application of Technology Platforms to the Purification of Monoclonal Antibodies, BioProcess International European Conference, Germany:11-14.
2.) Shukla, AA. 2007. Downstream Processing of Monoclonal Antibodies: Application of Platform Approaches. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 848:28-39.
3.) Sommerfeld, S, and J. Strube. 2005. Challenges in Biotechnology Production. Chem. Eng. Proc. 44:1123-1137.
4.) 2007 European Public Assessment Reports (EPARs) for Authorised Medicinal Products for Human Use. European Medicines Evaluation Agency; www.emea.europa.eu/htms/human/epar/a.htm.
5.) Nadeau, I. 2002. Low-Protein Medium Affects the 293SF Central Metabolism During Growth and Infection with Adenovirus. Biotechnol. Bioeng. 77:91-104.
6.) Durocher, Y. 2007. Scalable Serum-Free Production of Recombinant Adeno-Associated Virus 2 By Transient Transfection of 293 Suspension Cells. J. Virol. Meth. 144:32-40.
7.) Garcea, RL, and L. Gissmann. 2004. Virus-Like Particles As Vaccines and Vessels for the Delivery of Small Molecules. Curr. Opin. Biotechnol. 15:513-517.
8.) Štrancar, A. Freitag, R 2002.dvances in Biochemical Engineering Modern Advances in Chromatography, Springer-Verlag, Germany:49-85.
9.) Sellick, I. 2007. Capturing Large Biomolecules With Membrane Chromatography. Innov. Pharmaceut. Technol.
10.) Whitfield, RJ 2009. Rapid High-Performance Liquid Chromatographic Analysis of Adenovirus Type 5 Particles with a Prototype Anion-Exchange Analytical Monolith Column. J. Chromatogr.
You May Also Like






