Integrated PAT Automated Feedback Control of Critical Process Parameters Using Modern In Situ AnalyticsIntegrated PAT Automated Feedback Control of Critical Process Parameters Using Modern In Situ Analytics
November 17, 2017

Biomass measurement with BioPAT ViaMass system: Capacitance signal is proportional to viable biomass because only viable cells have undamaged membranes.
Simply put, the best way to control a critical process parameter (CPP) is to measure that specific parameter, integrate the live signal into your control system, and apply a smart feedback algorithm for an automated control loop. The challenge in doing this for bioprocesses has been due, in part, to the complex, highly dynamic, and variable nature of the process along with the lack of robust, scalable, and multiformat (single-use or multiuse) technologies that can monitor (in real time) such critical parameters (e.g., nutrients/metabolites and live cell concentration).
Other industries such as food and chemical have been conducting advanced automation of CPPs for decades. The apparent ease in which they do that may be due to the fact that those processes are binary or typically have a minimal number of changing components that are well defined. Ultimately, their success lies in the fact that robust and parameter-specific technologies have been widely available and well developed for this purpose.
A number of technologies and adaptations are now available for bioprocessing that are designed to help enable automation. Measuring CPPs with highly specific and scalable technologies that are adaptable to all bioreactor formats creates a strong foundation for advanced automation in bioprocessing. That is a critical point now because new bioprocess market dynamics and modalities are driving automation forward.
Online or In Situ Requirements
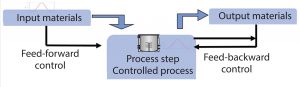
Figure 1: Process analytical technology (PAT) setup for realtime advanced analytics
For advanced bioprocess control, two key requirements must be met for online or in situ measurement. The first is to have a dedicated analyzer for each bioreactor providing “real-time” results. The real-time aspect is critical. (Here I refer to real time as having an in situ sensor measuring in seconds or minutes to provide rapid and robust feedback for fine control.) Second, a closed system for monitoring is needed to provide high sterility and no media volume reduction by sampling. That is key to robustness in manufacturing and successful development of control loops at small scale (Figure 1).
Such a setup provides several benefits for biomanufacturers. First, it can enable fine automated control. Continuous measurement also helps manufacturers control well-defined CPPs, thereby leading to product with consistent or improved quality. And from a regulatory perspective, automation improves patient safety.
It can be said that automation is not intended to keep processes at the same quality or throughput level as with manual operations. Rather, automation and real-time monitoring should improve processes with quality and yield gains as well as reductions in labor costs, risk, and waste. With online analytics, fewer offline samplings are needed, which gives operators time to perform other tasks. For a glucose-control loop, for example, sampling can be conducted automatically, and the human component is taken out of that process.
One example that ties in perfectly to this process analytical technology (PAT) strategy is autologous cell therapy, which is based on isolated T cells, lentivirus transfection, and administration back into patients. Monitoring for advanced parameters calls for looking at the cell expansion part of this process. For cell expansion, parameters such as glucose and viable cell volume are evaluated. The process is patient specific, highly variable, limited in working material, and it requires rapid timelines. Requirements include high sensor specificity — direct sensing of parameters that are to be tested in a closed system, which calls for in situ analytics.
Opening a bioreactor can create a batch failure or contamination risk. So automation should be applied where possible to limit the risk associated with human variability. In general, cell therapy processing can adopt PAT advanced analytics well, and such approaches can provide several benefits.
Offline Multiparameter Measurement with Autosampling
It is becoming common practice to integrate offline analyzers into bioreactor systems through an autosampling system. This is an easy transition because most process development laboratories already use offline analyzers. Such multiparameter analyzers can be connected to a bioreactor or fermenter through one of many autosamplers available on the market. That level of automation can be highly beneficial in terms of material throughput for process development. And online autosampling can be used to control nutrient feeds to some level and to monitor nutrients/metabolites and cell counts automatically. Having such an automated data collection system can significantly help with throughput because offline sampling is always a big bottleneck in process development.
Sartorius Stedim Biotech has integrated the Nova Flex 2 system with autosampling into its ambr15 system. This setup allows users to monitor multiple process parameters and obtain data at a relative frequency of every six hours. With this setup on a 24/48-way robotic microbioreactor system, design of experiment (DoE) studies can be conducted rapidly with some level of control.
Offline analyzers combined with autosampling will have limitations, particularly in terms of fine or low concentration control and with system scalability. In the context of process automation, the downside of conducting offline measurements with autosampling is that this system is difficult to implement into good manufacturing practices (GMPs). It can be a complex setup with cleaning, setup protocol calibration, and can introduce risk to a process. This type of system is not what I would consider “real-time” monitoring because of the relatively infrequent sampling and missing blocks of information. That can severely limit control and event automation possibilities. Could we use an offline analyzer with an autosampler for the fine control of glucose below a gram per liter? I don’t believe so.
Soft Sensors
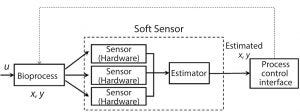
Figure 2: Soft sensors can contribute greatly to process visibility and operator guidance and are a big part of automation infrastructure.
Another component of process automation is soft sensors (Figure 2). They offer an indirect or predictive way to monitor and control. They are a big part of the overall automation infrastructure and can contribute significantly to process visibility and operator guidance. Soft sensors are inferential — conclusions are based on other process observations commonly used when hardware sensors for a specific parameter are unavailable. They play the role of surrogate for hard-sensor validation when performance declines through fault accumulation or drift.
Examples of soft sensors for monitoring/control in bioprocesses include glucose uptake rate, glucose control based on biocapacitance, and pH-based feed control. In general, soft sensors offer an indirect or predictive way to provide visibility or control. One interesting strategy is to use soft sensors to verify measurements from hard sensors or dedicated sensors (such as those for measuring glucose and lactate) or perhaps a Raman probe. In the context of advanced automation schemes, having as many sensors as possible verifying one another is a great approach. The typical downside to soft sensors, however, is that models tend to be complex and always highly susceptible to multivariate process variation.
Platform Integration “Plug and Play” Solutions for Fermentation Systems: Most people know what the term integration implies, but it has been a stumbling block and a general barrier for bioprocessing. If we provide sensors to customers, they can attempt to hook them up to their systems. But doing so requires company bandwidth, internal expertise on signal integration, and control loop design. That can create many bottlenecks and can increase the complexity or reduce the stability of a system. Off-the-shelf integration into unit operations and consumables allows easy and cost-efficient startup and routine operation without interface issues.
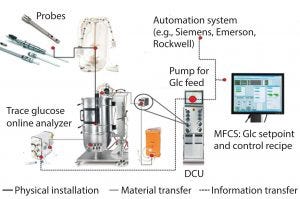
Figure 3: BioPAT platform integration; “plug and play” solutions for fermentation systems
Figure 3 shows an example of a BioPAT platform integrated control loop for glucose and lactate monitoring, including dialysis sampling. The analyzer is connected to a bioreactor, and output from the analyzer goes to the control tower. With the control tower, you can evaluate glucose and lactate in real time based on the analyzer signal. That signal is then taken to a software system, which applies a proportional–integral–derivative (PID) control loop. The setup provides continuous feedback so you are measuring or monitoring the glucose and controlling a pump with a PID controller. It is a holistic integration that can be tied into your plantwide system or historian software.
Within the control loop (typically for a glucose control loop where you are using PID control), the system provides a view of your controller, where you can modify your set point for glucose. Integration includes direct connection to a local controller, preconfiguration of that controller so that it displays the CPPs that you want to look at on one screen, and SCADA configuration included for advanced control and automatic calculations. Advantages of such a system include having a single interface for all sensors (classic and analytical) and the ability to automatically transfer data and conduct auto calculations and recipes.
Sartorius Stedim Biotech has developed a number of plug-and-play technologies for advanced automation in bioprocessing. Highlighted here are the BioPAT Trace technology for real-time glucose control and the BioPAT Viamass system for in situ viable cell volume monitoring and automation.
Glucose and Lactate Monitoring and Control
Most biopharmaceuticals are glycosylated proteins produced in cell culture and subsequently purified into drug substances before moving into a final dosage form. Glycation/glycosylation during cell culture can significantly affect stability and potency. Controlling media glucose to low concentrations and fine resolution during upstream processes can enable changes to ensure high product quality.
The ideal glucose concentration for a perfusion process is 0.1–0.5 g/L. Excessive available glucose can lead to inefficient or “lazy” cells and the increased production of lactate, which can reduce process yield. Perfusion processes never see a complete depletion of glucose because the glucose is replenished during perfusion. But at later stages and higher cell densities, it is common to need a supplemental glucose feed. Maintaining the glucose concentration at a low level for both perfusion and fed-batch processes can significantly improve yield.
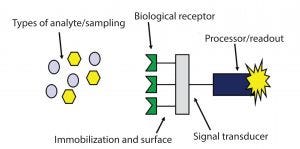
Figure 4: Components of biosensors
The BioPAT Trace analyzer provides an automated, on-line measurement and control of glucose (and lactate) with high specificity and low risk. It has the unique ability to control glucose feeding automatically at levels <0.5 g/L. It can lead to higher yields, improved quality, and reduced need for offline sampling. The system implements an enzymatic biosensor, which is similar to what you might find in an offline glucose analyzer (Figure 4). The biosensor uses glucose oxidase and lactate oxidase enzyme for an amperometric reaction when the analytes react with it. The BioPAT Trace analyzer is adaptable (for integration and scalability of advanced analytics) from 0.5 to 100,000 L and can be applied in multiuse stainless steel or glass and single-use bioreactors.

Figure 5: Single-system setup for perfusion or continuous process using BioPAT Trace and Multi Trace analyzers
Figure 5 shows a setup for perfusion control based on a glucose trigger and a trigger for bolus additions. This setup includes the analyzer (upper left of figure) and salt-based transport buffer (to bring analytes from the dialysis probe back to the sensor). Calibration standards keep the device maintained during long runs (e.g., 90 days for perfusion or continuous processes). The figure shows a single system, but a multisystem is available. The BioPAT MultiTrace system adapts to four vessels at a time, which can facilitate process development.
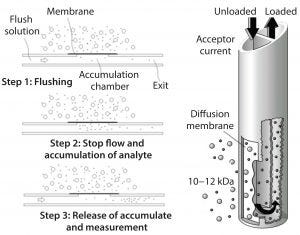
Figure 6: Dialysis probe technology
The dialysis probe shown in Figure 6 is the key technology component. Instead of filtering cell-free media from the bioreactor through the probe, it works through diffusion. A membrane at the end of this probe has a porosity of ∼10 kDa. During measurement, analytes pass through that membrane, are collected into the transport buffer, and carried to the sensor. So the probe can be used for small scales, measurements are taken up to once per minute without volume reduction. The process provides data density for fine control and fast response time — independent of media composition and viscosity. It works well for dirty microbial processes that might clog up (e.g., yeast). This type of sampling meets the challenging requirements for in situ and online bioprocess monitoring.
The BioPAT Viamass system works with a radiofrequency (RF) field probe. Cells react to this RF field based on their characteristics and their ability to hold a charge. A cell that has an intact membrane can be polarized with an RF field. When it is polarized, negative and positive ions align around the cell membrane, creating a microcapacitor. If a cell is charged, that charge can be measured to obtain a direct indication of the live cell mass in a bioreactor.

Photo 1: BioPAT ViaMass setup
The BioPAT Viamass system (Photo 1) contains a preamplifier that connects the controller to the sensor. A sensor disk is welded and presterilized directly into a rocking-motion or stirred-tank bag. This is an example of single-use adaptation of an advanced analytical technology whereby the application of capacitance technology, preintegrated into a single-use bag better enables single-use unit operations. In this case, single-use bioreactors need not be opened to install a probe. The next part is integration from a preamplifier into a controller, with the software built into the controller (everything integrated into one controller with one display).
Using Capacitance as a Monitoring Tool
Capacitance technology has applications in both upstream and downstream operations and has been shown to be a robust tool for multiuse and single-use unit operations. The signal is “visualizing” cell growth quite simply, allowing the direct monitoring of cell growth. The signal can be used for feed control, perfusion control, or for harvest-point detection. For downstream processes, the BioPAT Viamass system can be used to determine the amount of filtration material needed (with number of filtering cassettes and pH adjustments).
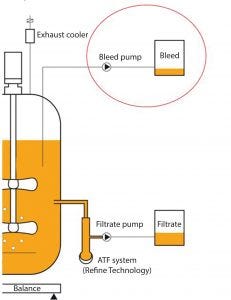
Figure 7: “Cell bleed” for continuous bioprocessing; cells are removed from the bioreactor through a dip tube using a peristaltic pump at a defined flow rate. The cell-bleed rate is equal to the growth rate to maintain a steady cell density.
One interesting application is using capacitance to correlate to an offline technology, which is typically a trypan blue method in which cells are stained and counted. Offline and online technologies look at slightly different factors: One is total cell count, and the other is viable cell volume. In the industry, there can be some stumbling or tripping over that concept, but many companies have adopted capacitance technology — the raw form of the capacitance signal — for process control. The main reason here is that capacitance is by far the most robust way to monitor cell growth in a bioreactor without the need to pull a sample out of a bioreactor and taking it to an offline analyzer for analysis and all the pitfalls in that process.
Figure 7 shows a cell bleed, which is the removal of cells from a bioreactor. It includes a dip tube in a bioreactor, a peristaltic pump, and a defined flow rate. The cell bleed rate is determined by the cell growth rate, and the cell density may be limited to a desired value in a continuous flow loop. The cell bleed rate is equal to the growth rate to maintain a steady cell density. This is an easy application for the BioPAT Viamass analyzer because it is monitoring live cell volume in real time.
In summary, dedicated, real-time, on-line sensors give provide a robust system for controlling your bioprocesses, significant risk reduction, and finer control possibilities. Such PAT advanced analytics coexist and are complementary to soft sensing and autosampling systems. They set the foundation for advanced process control strategies using multivariate data analysis.
Dan Kopec is a process analytical technology (PAT) expert for Sartorius Stedim Biotech in North America, 5 Orville Drive, Bohemia, NY 11716. He has over 15 years with Sartorius Stedim and 20 years experience in process centers for monitoring control and automation in the biopharmaceutical, food, and chemical industries.
This article was based on a presentation at Biotech Week Boston, 24–28 September 2017.
You May Also Like






