Disposable Downstream Processing for Clinical ManufacturingDisposable Downstream Processing for Clinical Manufacturing
Although disposable parts and modules have been used in the biopharmaceutical industry since the 1970s, as detailed in the “History” box, total disposable manufacturing has become a viable option only very recently. Whereas liquid storage became disposable in the 1990s, processing operations such as depth filtration, tangential-flow filtration (TFF), and chromatography have still required skids with reusable flow paths that needed cleaning and sanitization. Important recent milestones in total disposable technology included introduction of stirred bioreactors by HyClone (Thermo Scientific) in 2004; depth filtration in 2005 and TFF in 2009, both by Millipore; and chromatography by GE Healthcare in 2009.
At small to medium scales, single-use technology offers several advantages over traditional stainless-steel manufacturing technologies: reduced capital expenditure, shortened engineering and qualification timelines, and reduced maintenance. For operation in multiproduct facilities, disposable equipment provides increased flexibility (with regard to raw materials and cell systems) and presumably faster turnaround times.
Because acceptance of single-use technology for clinical manufacturing has significantly increased during the past few years, Rentschler decided to design and operate two 1,000-L scale cell culture good manufacturing practice (GMP) lines for contract manufacturing based totally on disposable equipment.

After completing the engineering phase and progressing into construction and qualification, the company gained significant benefits: Capital spending and expenses are likely to be reduced to 60% of a comparable-scale facility based on conventional manufacturing technologies. Overall project timelines will be shortened by eight months, which amounts to a 30% reduction from what it would have been with reusables.
A BRIEF HISTORY OF BIOPROCESS DISPOSABLES
Single-use manufacturing technologies have been used in the bioprocess industry for over 30 years.
1970s: Membrane filters, blood bags
1980s: Virus filtration (Asahi Kasei)
1990s: Bags for buffer and media storage and transportation
1991: Membrane chromatography (Sartorius)
1999: WAVE bioreactor (Wave Biotech, now GE Healthcare)
2004: 250-L stirred-tank bioreactor (HyClone/Thermo Scientific)
2005: Depth filtration skid (Millipore)
2009: TFF filtration skid (Millipore)
2009: Chromatography skid (GE Healthcare)
The most crucial factor for realizing those savings was elimination of clean-in-place (CIP) and steam-in-place (SIP) utilities, which significantly affected engineering, capital spending, and qualification efforts. That led to a significant reduction of overall utility needs. The disposables-based manufacturing facilities require only process gases and cooling water. Major additional cost savings resulted from eliminating a disk-stack centrifuge from process unit operations, instead using only filtration for harvesting (Figure 1).
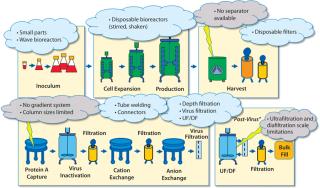
Figure 1: ()
Rentschler follows an animal-component–free and virus-free policy for all stainless-steel GMP manufacturing lines. “Total disposable” manufacturing will allow processing of serum-containing media (e.g., for legacy processes). More important is that it will enable the company to operate multiproduct facilities for manufacturing virus-based biologics to be applied as vaccines and for gene therapy.
Disadvantages of current single-use technologies include volume limitations for some unit operations (e.g., TFF and chromatography), less automation capability, and certain limitations of closed processing in downstream operations. Figure 1 overviews the current state of single-use technology for bioprocessing and its application for monoclonal antibody (MAb) manufacturing.
Perceived risks include leachables/extractables and leakage and contamination due to robustness problems with some bags and assemblies. Experience still needs to be gathered and published regarding scalability and transferability among both disposable systems and conventional equipment. To ensure robust and scalable manufacturing, Rentschler carried out two concept studies dealing with upstream and downstream technology for MAb manufacturing. Upstream (Production) Study
The scope of the upstream study included characterization of stirred disposable bioreactors and depth filtration skids (1). The objective was to choose the best-suited process equipment for Rentschler’s new facility. The company tested systems by cultivating a MAb-producing CHO cell line according to a high–cell-density platform process protocol. Comparability was assessed based on physical equipment parameters as well as metabolic parameters, product quality, and operability. Test runs at 200-L scale in bioreactors from HyClone, Xcellerex, and Sartorius Stedim as well as in a conventional stainless-steel bioreactor showed adequate reproducibility of process parameters and suggested good transferability among all systems. Furthermore, product quality was comparable for all cases. Filtration studies of disposable dead-end filtration systems for cell separation from Cuno, Millipore, Pall, and Sartorius Stedim demonstrated improved performance and reduced costs for disposable harvest filtration compared with the combination of disk-stack centrifuge and depth filtration routinely used in conventional facilities. Process intensification by omission of centrifugation showed good yields and seems generally feasible. However, technology transfer between filtration only and centrifugation–filtration still needs to be investigated for large-scale processes. Downstream Processing Study
The downstream study overviews total disposable availability as well as current capabilities and limitations of single-use technology. Because downstream disposable technology is developing quickly, this report cannot be regarded as an exhaustive study. To address issues of leachables, Rentschler is currently carrying out an in-depth risk assessment for all disposable materials used at the facility. Contact us directly for more information.
Downstream Total Disposable Manufacturing Technology: Current capabilities and limitations of available downstream technology are evaluated with respect to a 1,000-L scale generic fed-batch MAb process with a titer of 1–3 g/L. It is assumed that the process is at clinical manufacturing stage, so only a few product batches are needed. This model process comprises protein A capturing, virus inactivation, cation-exchange (CEX) chromatography, anion-exchange (AEX) membrane chromatography, virus filtration, and ultrafiltration/diafiltration (UF/DF) for fo
rmulation of the bulk drug substance.
Chromatography: With its ÄKTA ready system, GE Healthcare is currently the only supplier offering a disposable chromatography skid. (Millipore is also developing a system, but only prototypes are yet available.) With a maximum volumetric flow of 510 L/h, the ÄKTA ready system is designed for column volumes up to 20 L. At 1,000-L scale, that means running multiple chromatography cycles for capture and CEX chromatography. The procedure is acceptable for clinical manufacturing because it helps reduce overall protein A resin costs — a dominant cost factor at several hundred-thousand euros per column. For commercial manufacturing at 1,000-L scale, however, with more than a few batches and long column lifetimes, this scenario is no option. With small column volumes, the limiting parameters for downstream operations here would be intermediate hold times and overall process times rather than cost of goods. Figure 2 shows the overall processing scheme based on available equipment sizes. With two shift operations, this would require eight days.
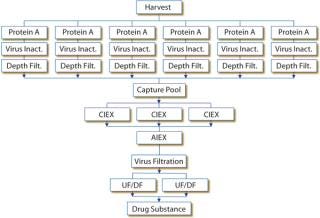
Figure 2: ()
The compact GE system is designed for simple, robust operation. Its flow-path assembly can be exchanged within minutes and allows fast turn-around times. It remains to be seen whether the flow path can be cleaned and sanitized for multiple consecutive cycles of a single batch or whether it needs to be replaced for every run. A severe limitation is the lack of a gradient system — not so much for performing gradient elutions, but rather for inline dilution, which would help reduce buffer volumes significantly for both column and membrane chromatography operations. GE is currently working on implementing a gradient system, which should be available in 2012.
Apart from the omission of cleaning and cleaning verification/validation, another significant benefit of prepacked columns comes from the outsourcing of packing and packing validation to column manufacturers. Such work typically amounts to 5–10% of all preparation work in downstream processing. GE sells prepacked columns at 1-L to 20-L scales. EMD Millipore has developed prepacked columns too. However, both companies will provide only their own resins. To obtain other chromatographic media in prepacked columns as well, Rentschler is currently working with Atoll GmbH in Weingarten, Germany (a provider of 96-well format and laboratory- to pilot-scale columns), on scale-up for clinical manufacturing.
Tangential-Flow Filtration: Disposable TFF units are currently available up to 2.5-m2 scale from Millipore, Pall, and Scilog. Sartorius Stedim offers total disposable systems only up to 0.3 m2 in scale. No larger system is currently available with the ~5-m2 membrane area that would be needed for efficient one-cycle-only processing at 1,000-L scale (Figure 2). So TFF constitutes a bottleneck not only for commercial manufacturing, but also for clinical manufacturing with disposables.
Rentschler tested filtration skids from both Millipore and Pall. With its membrane pump — rather than the peristaltic pump included with the Millipore Mobius system — the Pall skid can generate about twice the volumetric flow per square meter of membrane. Dead volumes are comparable for both systems. Whereas the Pall system is fully automated, the semiautomated Millipore system is equipped with manual valves. Table 1 lists specifications for both skids in comparison to Rentschler’s 1,000-L scale requirements.Table 1: Tangential-flow filtration specifications
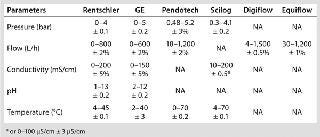
Table 2: Specifications of selected disposable sensors ()
No UF cassette modules are available gamma-sterilized, so module sanitization still accounts for 15–20% of overall TFF process time. Unfortunately, the mode of plug (equilibrate) and operate that has become common for much disposable processing, does not yet apply for TFF.
Virus Filtration: Because no standard systems were yet available at the 1,000-L scale, Pall customized a system for the Rentschler facility that can hold up to four modules with 1–16 m2 of membrane area. This skid is fully automated. Virus filtration modules are connected through sterile connectors to a gamma-sterilized flow path assembly, which also includes prefilters (Figure 3).
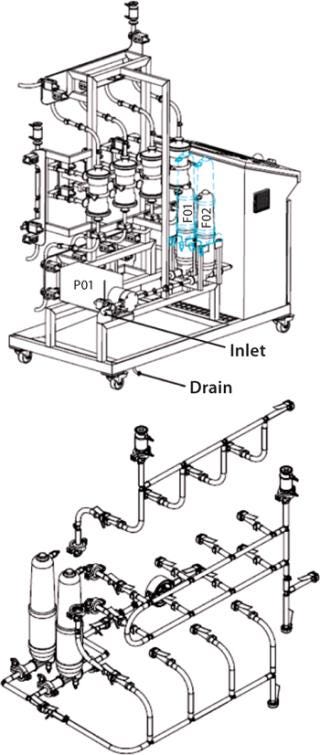
Figure 3: ()
Mixing Systems: By contrast with the downstream unit operations described above, disposable mixing skids are well established and available from diverse suppliers up to 1,000-L scale. Manufacturing teams at Rentschler have ample experience with both the Sartorius Stedim and Millipore mixing systems, so we compared both with an ATMI Integrity PadMixer system. Although our study revealed no significant differences in mixing capabilities, we found that heat-transfer capabilities varied among the units. Both the Sartorius Stedim and Millipore skids achieved temperature shifts from 5 °C to room temperature and back within three hours, but the ATMI system needed five hours to do the same. Preparation of the ATMI system is more complex compared with the Millipore and Sartorius Stedim systems. Figure 4 summarizes the criteria we assessed in this study.
Figure 4: ()
Tube Welding and Sterile Connectors: Because tube welding can be applied conveniently throughout all upstream manufacturing operations, it clearly is the technology of choice — except when introducing conventional probes into bioreactors. However, downstream operations provide a somewhat different picture: Use of tube welding is limited by several factors.
First, due to limited accessibility, several tubings of
existing flow-path assemblies would have to be extended. That could compromise the need for low dead volumes. Second, customization of all standard flow-path assemblies (which do not include C-flex ends for welding) would create additional cost and supply chain issues. And finally, making multiple connections and disconnections during operations (e.g., for chromatography) could take significantly more time when applying tube welding. Reliable sterile connectors are available in different designs from different companies up to 1-in. tube diameter sizes, so Rentschler decided to apply different strategies in upstream and downstream processing. Tube welding and customized assemblies and manifolds were implemented for upstream production; connections in downstream processing are made either using sterile connectors or manually under laminar-flow hoods. Work in Progress
Development of “total disposable” upstream production manufacturing technology is several years ahead of that for downstream process technology. Most process limitations and bottlenecks currently occur downstream. The gap between up-and downstream could even worsen as production titers coming out of process development increase. However, single-use technology currently is an attractive option for clinical manufacturing up to 1,000-L scale. Benefits can be leveraged with regard to capital spending, implementation times, maintenance reduction, improved turnaround times, and increased flexibility regarding cell lines, raw materials, and products. Suppliers are innovating and working to provide solutions for the bottleneck problems even as we write this.
About the Author
Author Details
Dr. Markus Laukel is associate director of protein chemistry ([email protected]), corresponding author Peter Rogge is director of protein chemistry ([email protected]), and Dr. Gregor Dudziak is vice president of cell culture ([email protected]) at Rentschler Biotechnologie GmbH, Erwin-Renschler-Straße 21, 88471 Laupheim, Germany; www.rentschler.de
REFERENCES
1.) Dudziak, G.. Full Plastics: Generic Antibody Manufacturing Using Total Disposable Technology.
You May Also Like





