Characterization-Based Manufacturing Process Development of Protein TherapeuticsCharacterization-Based Manufacturing Process Development of Protein Therapeutics
July 1, 2009

KBI Biopharma is a leading contract development and manufacturing organization for the biopharmaceutical industry. We accelerate and optimize drug development and manufacturing programs for our client partners, including global pharmaceutical and biotechnology companies and organizations in biodefense. Our clients benefit from faster time to market and lower cost of manufactured goods.
Ongoing Innovation
We are continually employing new technologies for the analysis of proteins and developing new partnerships, technologies, and services to enhance biomanufacturing. Our expertise in biophysical and biochemical protein characterization makes us highly successful in delivering optimal product stability and bioactivity for our clients. We achieve this through our ability to integrate the characterization data into the manufacturing process and formulation development.
Proven Approach
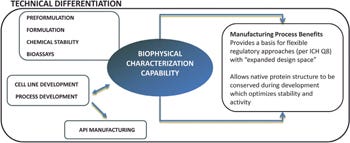
Biophysical characterization early in the process can accelerate the development trajectory of the molecule. Through this characterization, we can select the optimal manufacturing processing conditions and media formulation to yield the highest quality protein, and we can also select the best host organism for the molecule. By fully understanding the protein, we can develop a process strategy to optimize each step — from expression through final formulation — resulting in superior process yields and a high-quality, maximally potent molecule. Furthermore, biophysical characterization is critical to the development of optimized, robust processes and stable, effective formulations that allow for lower costs of goods and decreased regulatory burden.
For example, during biophysical characterization, we determine the effect of pH on the thermal, conformational, and physical stability of the protein. We can then apply that information during upstream and downstream manufacturing process development, reducing the potential for pH-related denaturation and degradation that negatively impacts process yield and product quality. We have a proven track record in formulation development, analytical development, and protein characterization, and we have expanded our capabilities in other areas of biopharmaceutical product development such as cell line generation, process development, clinical manufacture, and GMP release and stability testing.
The KBI Difference
Too often, formulation development is employed to “fix” a protein after it has been subjected to harsh manufacturing processes. Our approach allows us to design processes suited to maintain the native conformation of the protein at all stages. This conservation of native structure is expected to result in optimal product stability and potency. We believe that our ability to fully understand the molecule creates a value that is unmatched by other contract development and manufacturing organizations.

Scientific Expertise
Our scientists have extensive expertise in the development of proteins, peptides, antibodies, and vaccines. Our team uses specialized understanding of biopharmaceutical and pharmaceutical development and commercialization to deliver a high-quality technical package to our customers. Our scientific, quality, and regulatory staff possesses the experience — proven through numerous IND and NDA submissions — to meet the challenges that arise at all stages of drug development. The advantages we offer our customers stem from our knowledge of the key issues they face: the need to develop and commercialize biopharmaceuticals that will dramatically improve the quality of life in the 21st century — products that have to get to patients as quickly as possible while proving to be economically viable. By clearly defining the design space possibilities associated with a particular molecule, our clients gain a road-map of opportunities for future growth.
Comprehensive Services
Our service offerings can take you from clone selection through supply of clinical trial material — which gives you control and predictability in planning and budgeting, faster time to the clinic, and seamless technology transfer to larger-scale manufacturing. We provide our services using robust internal processes and quality systems, ensuring that you receive a quality technical package that meets or exceeds your expectations.
Extensive Facilities and Capabilities
Our laboratories are cGMP-compliant and equipped with state-of-the-art instrumentation to ensure rapid and accurate product characterization, method development, and cGMP data generation. Our spacious facilities provide additional avenues for collaboration.
KBI services include the following:
Cell line generation in mammalian, insect, bacterial, and yeast expression systems
Cell culture, fermentation, and purification process development
GMP manufacturing for Phase I, II product
Cell culture (manufacturing to 2,000-L working volume in single-use bioreactor systems)
Fermentation (100 L working volume)
Biophysical and analytical product characterization
Analytical method development
Formulation development
Stability programs
Client Commitment
When you choose KBI Biopharma as your drug development partner, you can expect individualized attention, excellent communication, and ongoing collaboration with a study director and senior scientists working on your project. At KBI Biopharma, we offer services that enable you to steer your way through critical stages in the process — productively, efficiently, and with the greatest opportunity for long-term success. We recognize the incredible investment you have already made to bring your products to this point, so we make it our goal to become a seamless and responsive extension of your own organization and take the output of your work to the next level.
You May Also Like






