How to Balance High-Titer Processes with Consistent Quality?How to Balance High-Titer Processes with Consistent Quality?

The high cell culture process (XD®) is a continuous process in which both cells and product are retained in a stirred-tank bioreactor using suspension mammalian culture. This is accomplished using a retention system: Fresh medium is continuously supplied, and metabolic byproducts are withdrawn and discarded through the retention system. XD® provides a controlled environment that leads to consistent product quality in terms of bioactivity, glycosylation pattern, and other product characteristics. Moreover, XD® technology is independent of the cell line (e.g., CHO, PER.C6®, hybridoma, myeloma). Developments have led to very high viable cell densities of >150 × 106 cells/ml and IGG concentrations of 10–27 g/L in a period of 10–14 days. In the case of CHO cells, XD® delivered 8–13 times higher titer than comparable fed-batch runs. With the XD® technology the volumetric productivity of the bioreactor is boosted to very high levels and can ideally produce large amounts of product with minimum development and investment. It is also suited for manufacturing nonantibody proteins, which can be difficult to produce at high titers in traditional fed-batch systems due to limited cell specific productivity. XD® delivers consistent product quality and titer during scale-up, and its application in cGMP manufacturing opens new avenues for biologic therapeutics production.
Case Study
The original CHO-based 12-day fed-batch process yielded 1 g/L of a fusion protein. Early trials applying XD® technology combined with DSM’s active experience in biologics manufacturing resulted in eightfold improvement in titer using the same medium. Further process development included optimization of the feeding strategy, media component, and process parameters. Such developments resulted in extremely high cell density of 175 × 106 cells/mL on day 12 (Figure 1). Product concentration was improved eightfold over a fed-batch run at the same duration (12 days, Figure 2).
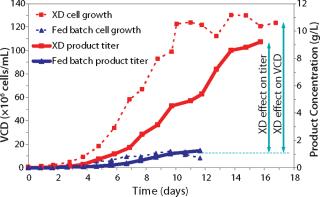
Figure 1: ()
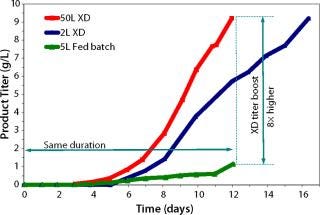
Figure 2: ()
Scale-Up: The process was further scaled up to a 50-L bioreactor. Continuous optimization and scale-up of the process resulted in consistent high titers at the 50-L scale (Figure 2). In addition, the culture maintained a high viability (>95%) right up to the point of harvest. This can potentially facilitate downstream clarification and purification. the use of XD® technology to achieve an eightfold titer increase compared with the original 400-l fed-batch decreases the plant footprint and extends related equipment and facility capabilities. Finally, because the product is collected inside the bioreactor within the same time frame of a standard fed-batch process, only a small and concentrated batch is harvested. Therefore, the need to collect and purify large, multiple-volume harvest batches is eliminated. A similar process was transferred for the cGMP area for further manufacturing and scale up.
About the Author
Author Details
Rolf Douwenga is vice president of global R&D; Gerben Zijlstra, PhD is a senior scientist in R&D; and Alberto D’Avino, PhD, and corresponding author Amir Goudarzi, PDEng, are associated scientists in USP R&D, DSM Biologics, Zuiderweg 72/2, 9744 AP Groningen, The Netherlands, 31-505-222-389; amir.goudarzi@dsm. com, www.dsmbiologics.com.
You May Also Like






