Optimization and Scale-Up of a Downstream Antibody Process Using a High-Throughput ApproachOptimization and Scale-Up of a Downstream Antibody Process Using a High-Throughput Approach
July 1, 2009

Figure 1:
The recent successful production and application of new therapeutic antibodies has increased the demand to develop the next generation of antibodies. The race to develop new antibodies requires excellence in process development and full-scale production. Following the discovery phase, potential drug targets are taken from early clinical phases to market approval. During this process, efficient screening tools are important for the exploration of the experimental design space — in line with US FDA quality by design (QbD) initiatives — and subsequent optimization of the downstream protocol to develop robust and high-yield processes in a short time.
In this case study, the primary goal was to develop a robust and highly productive downstream purification protocol for a challenging antibody feedstream containing high levels of aggregates. The secondary but equally important goal was to scale up the optimized process conditions for the two chromatographic steps in a short time, thereby meeting the pre-defined purification targets set from the small-scale experiments. The downstream process was based on a two-step chromatographic process using MabSelect SuRe™ for the capture step and Capto™ adhere for the second and final polishing step (Figure 1).
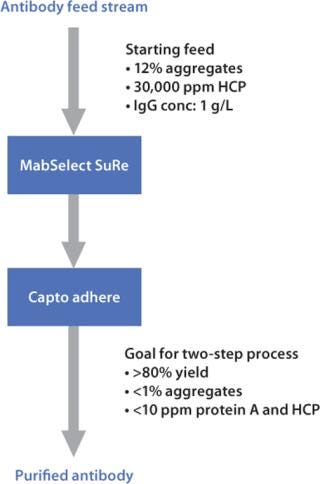
Figure 1: ()
The workflow was based on three steps:
High-throughput screening of different process conditions in PreDictor™ 96-well plates for a rapid and broad evaluation of process conditions for the capture and polishing steps
Verification and optimization of the most promising process conditions for each chromatography step in prepacked HiScreen™ columns
Development of a full-scale process through a combination of design of experiments (DoE) and Monte Carlo simulations where the DoE results were tested for robustness by the simulation studies.
The final scaled-up process used AxiChrom™ 70 columns with intelligent packing based on time-saving protocols.
Screening and Optimization of the Protein A Capture Step
Using PreDictor 96-well plates prefilled with MabSelect SuRe media, a total of 480 experimental conditions were tested in two days. Studies included conditions for binding and elution. Detailed investigations were performed on the effect of incubation time on antibody binding and on how protein concentration, different additives, conductivity, and pH affect purity and yield. In a subsequent optimization phase, selected conditions from the PreDictor 96-well plate experiments were verified and optimized in prepacked HiScreen columns. Results from the column experiments were combined into a sweet-spot analysis plot with pre-set criteria for the capture step met (Figure 2A).
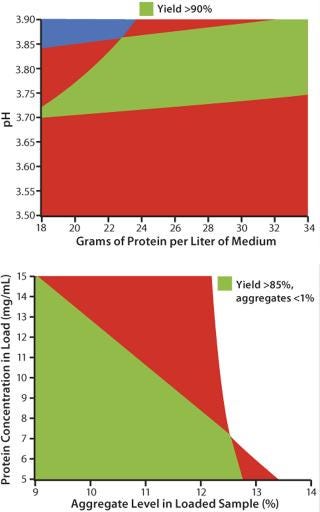
Figure 2: ()
Screening and Optimization of the Polishing Step
This polishing step is run in flow-through mode using Capto adhere, a multimodal ion exchanger specifically designed to bind aggregates, leached protein A ligand, and host cell proteins. The purified antibody does not bind to the column but is found in the flow-through, resulting in a two-step process. Following the workflow described for the capture step, 192 experimental conditions were tested over two days for binding and elution. Detailed investigations were performed for the removal of aggregates, host cell proteins, and leached protein A ligand, as well as for antibody yield. Optimization and verification were performed on HiScreen columns prepacked with Capto adhere media, resulting in a sweet spot analysis plot where the preset goals were achieved (Figure 2B).
Scaling Up the Optimized Antibody Process from HiScreen to AxiChrom
A thorough screening and optimization process at small scale generated valuable information on critical process parameters for the process steps, allowing for the design of a scalable and robust process meeting the set quality attributes. The process conditions for the capture and polishing steps shown in the sweet spot plots in Figure 2 were further analyzed (in terms of process robustness) by Monte Carlo simulations. For the capture step, four different factors were investigated by these simulation studies: antibody load, NaCl concentration in the wash, elution flow rate, and elution pH. For the polishing step five different factors were analyzed with respect to robustness: aggregate levels, antibody start concentration, antibody load, NaCl concentrations, and pH for elution.
Simulation of the capture step predicted a yield of 94.7–96.0%, whereas simulations for the polishing step predicted a monomer purity of 99.2–100% and yield of 82.5–86.7%. In the scaled-up process, transferring from the 5-mL scale in HiScreen columns to MabSelect SuRe and Capto adhere packed in AxiChrom 70/300 at the liter scale, near identical results were obtained for the process (Table 1).
Table 1: Comparative results at small scale (5-mL HiScreen columns) and the scaled-up processes performed at liter scale in AxiChrom columns

Table 1: Comparative results at small scale (5-mL HiScreen columns) and the scaled-up processes performed at liter scale in AxiChrom columns ()
Summary
In conclusion, a successful two-step downstream purification process for a challenging antibody feed containing high aggregate levels was achieved. The process conditions were screened in PreDictor 96-well plates filled with MabSelect SuRe for the capture step and Capto adhere for the polishing step. Process conditions were verified and optimized in HiScreen columns before evaluation of the process for robustness by Monte Carlo simulations. The scaled-up process on AxiChrom columns at the liter scale gave near identical results. The use of PreDictor plates in such screening studies provides rapid results over a broad range of test conditions. Such results not only accelerate the purification process, but also provide critical data for a better understanding of the process as prescribed by the QbD initiative.
©2009 General Electric Company – All rights reserved. AxiChrom, Capto, HiScreen, MabSelect SuRe, and PreDictor are trademarks of GE Healthcare companies.
You May Also Like






