Providing Process Solutions for the Biopharmaceutical Industry WorldwideProviding Process Solutions for the Biopharmaceutical Industry Worldwide

Figure 1.
Integrating production processes within a complete facility does not always lead to a costly execution and long time to market for end users. This is what a specialist company such as Boccard can offer thanks to its expertise in process design and construction of pharmaceutical GMP systems, and thanks to a different project implementation strategy that focuses on the client’s core business, production needs, and operating methodologies.
Case Study: Alpha Biologics Facility Project in Malaysia
Present in 20 countries, with 3,000 employees worldwide and more than 30 years of excellent track record as a turnkey process system supplier, Boccard has become a major player in the pharmaceutical and biotechnology industry.
Alpha Biologics is an independent CMO offering FDA/EMEA CGMP compliant services to the worldwide pharmaceutical and biotechnology industry. Initial process development is undertaken by their experienced team in Cambridge, England, before being transferred to Malaysia for final process technology transfer and manufacturing of biologics drugs for clinical trials.
The 5,000-m2 facility, planned to produce mammalian cell secreted proteins—including monoclonal antibodies and recombinant proteins—has been designed by a team involved in numerous CGMP compliant facilities, many of which have undergone successful EMEA/FDA audit and are fully approved facilities. Boccard and alpha Biologics teams submitted a design review package to CBER FDA that declared the facility suitable for both clinical trial and early market product manufacture.
After concept design completion, basic design has been undertaken to finalize the facility specifications, splitting the project into three main scopes of supply: building, cleanrooms and HVAC, and process and utilities. Boccard has played the role of general contractor as well as process systems and utilities supplier.
Our Approach: A Successful Path to Completion of Your Project
The innovative project implementation strategy benefits clients who are searching for rapid time to market as well as clients in developing countries where biotechnology or Western standard GMP pharmaceutical facilities are still considered “new” hightechnology or high-standard degree.
Boccard’s centralized pharmaceutical engineering department, with internationally experienced personnel, has heavily capitalized on GMP process expertise and has established a working methodology enabling successful performance worldwide.
As a specialist company, Boccard has dedicated pharmaceutical resources to design, build, and validate complete turnkey GMP facilities, providing clients with valuable engineering and a design-construction synergy oriented toward construction and validation stage.

Additionally, as a process system supplier, Boccard is capable of designing facilities “from inside to outside” by ensuring that clients have proper functional spaces for their specific processes. Value has been added with the integration and appropriate interfacing between the process, buildings, utilities, HVAC, and cleanrooms.
The initial focus is on the equipment in contact with the product (e.g., bioreactors, ultrafiltration, chromatography). The area around the equipment must be practical for installation, operation, and maintenance. Therefore, a precise specification of the equipment dimensions must be known up front to optimize the facility layout.
The secondary focus is on less critical equipment or infrastructure (e.g., clean rooms, CIP systems). They must provide an ancillary support around the process (e.g., air classification, cleaning), but they also bring constraints in design (e.g., space for low return air, pipe length with turbulent flow) that can impact the layout drastically.
The last focus is on the building and engineering utilities, which have to be sized to house the complete manufacturing operations plus all the related activities (e.g., warehouse, laboratories, clean utilities area, offices).
The utilities sizing must be done using a simulated production Gantt chart to evaluate the utilities peak consumption and diversity. The utilities are paramount issues for a facility, and inappropriate design would soon create a bottleneck in the production.

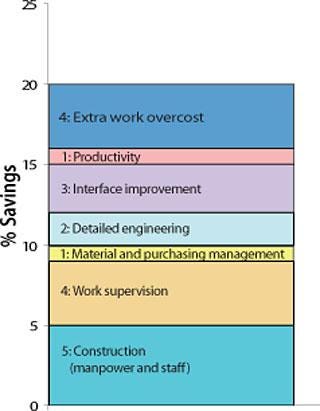
Figure 1. ()
Project performance is increased through skid-mounted equipment solutions, and quality is ensured by a stringent quality control workshop. Equipment assembly becomes easier, safer, and faster within a totally accessible working space. Before site delivery, the equipment will undergo comprehensive factory acceptance tests (FAT) almost reproducing the qualification performance tests.
The Boccard workshop is fitted with all the necessary utilities required to extensively test the skids. Therefore, the FATs will simulate all the equipment functionalities. Any skid-mounted solutions must be primarily compliant with URS functionalities before being declared standardized or customized.
Boccard has identified the need for clients to be fully supported during the facility production start-up and always proposes to relocate an expert to the client site during one year after validation. The expert’s task is to train the client’s operating and maintenance personnel and provide general assistance in an effort to optimize and rapidly troubleshoot the facility.
Benefits of Our Solution
The approach is time saving because of the limitation of interfacing and therefore the reduction of potential dispute between subcontractors. As a process and utilities supplier, Boccard is capable of shortening the equipment supply delivery schedule to reduce and secure delivery critical path.
Clients recognize that specialist companies provide qualified means to undertake projects, mobilize resources at project startup, and ensure that the synergy between design and construction will shorten the project delivery schedule by at least 10%.
Because of ever-increasing R&D cost, capital expenditure in a new facility has to be wisely evaluated before the green-light is given. The design and build solution through a specialist company is providing a capital savings of
about 20% as a result of value engineering, limitation of interfaces, detailed engineering, and use of local contractors.
As drug producers, pharmaceutical companies expect to validate and run production facilities, and therefore end users do not expect to bear the design and construction risks.
As a specialist company, Boccard is able to provide a genuine turnkey solution in which the general contractor (and not the client) is bearing the risk of any budget slippage and time delays.
You May Also Like






