- Product Characterization
- Biosimilars
- Sponsored Content
Biosimilarity Assessments: The Totality of Evidence FrameworkBiosimilarity Assessments: The Totality of Evidence Framework
Sponsored by 4Tune Engineering
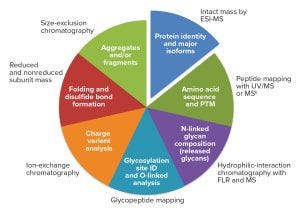
Figure 1: The analytical package; methods used for similarity and comparability assessments for biotechnology products (ESI-MS = electrospray ionization mass spectrometry; FLR = fluorescence)
Biosimilars are evaluated through comparisons with their reference products using abbreviated pathways that have evolved significantly over the past few years. Scientists and regulators now accept that some quality attributes can vary from batch to batch over a product’s lifecycle, even for reference products. Moreover, reference and similar biotechnology products can show differences in noncritical quality attributes but still demonstrate comparable efficacy and safety (1).
Here we describe a similarity assessment approach that is also applicable to comparability of lifecycle assessments. It falls in line with the US Food and Drug Administration’s (FDA’s) totality of evidence concept (2) and uses the entire analytical package generated for each biotechnology product (3, 4).
Analytical Package
Product characterization involves a comprehensive set of analytical techniques that, as a whole, define an analytical package (Figure 1). This means that different types of data will need to be considered holistically for multiple quality attributes from several analytical techniques, along with a complex exercise linking variability in quality attributes related to clinical performance (safety and efficacy). Furthermore, for a similarity assessment (between a similar and a reference biotechnology product), two analytical packages must be compared in terms of quality attributes that have a causal link to clinical performance (critical quality attributes, CQAs). A large number of practical aspects conspire, however, to make such one-to-one CQA assessments a daunting
task: from the robustness of bioanalytical-clinical causality, to challenges related to unambiguously and accurately measuring each CQA, to making sure all clinically relevant CQAs are accounted for. It is easy to understand why reliable assessments are very difficult to perform using classical statistical approaches.
The Totality of Evidence Framework:
Figure 2: Totality of evidence (TOE)-based pathway to demonstrate biosimilarity to reference product
According to the US FDA, the evaluation and approval of an interchangeable biosimilar product should be based on robust and comparable bioanalytical and clinical packages, a totality-of-the-evidence approach. A submission for approvalshould provide an overall assessment that the proposed product is biosimilar to or interchangeable with its reference product (Figure 2).
Four key concepts contribute to performing that assessment:
abbreviated clinical pathways that are followed for biosimilar development because establishing safety and efficacy of a new product is different from demonstrating biosimilarity to a reference product
evidence-based development that demonstrates biosimilarity, with evaluation of residual uncertainty at each step of product characterization studies (analytical and clinical)
extensive analytical similarity, an extensive structural and functional characterization on which to base the assessment, a well-rounded product/process quality-by-design (QbD) foundation for the similar product, with a robust manufacturing science and technology package ensuring a reliable commercial operation over its lifecycle
clinical studies that must support and not disprove similarity, be commensurate with similarity levels observed from the two analytical packages assessments, and consider the residual uncertainty between the products (e.g., fingerprint-like similarity and residual-risks).
Similarity and Comparability Assessments – A New Approach
Aligned with the concepts of fingerprint-like similarity and residual risks (2), this new approach proposes using the entire domain of each analytical technique used, extracting what is relevant from each domain to produce a method’s fingerprint. All the information from an analytical package is aggregated to produce a multimethod design space for each product (a multivariate specifications nominal operating ranges space, NOR). The outcome of such an approach is a comprehensive and quantitative assessment of both matches (such as fingerprint-like similarity) and mismatches (such as residual-risks) in the assessments, with full traceability to an individual method and specific analytical domain feature implicated – e.g., the individual quality attribute (Figure 3).
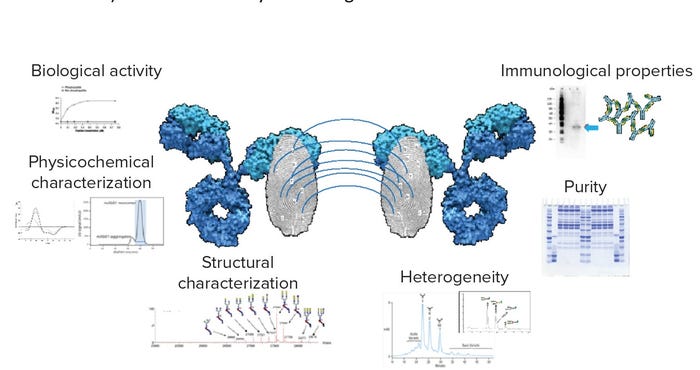
Figure 3: Fingerprint-like approach and totality-of-evidence concepts introduced by the FDA when establishing similarity or comparability assessments
This approach is applicable to similarity assessments (product-based). Also, assessing method fingerprint variability can enable detection of process performance shifts that normally compromise product quality – a key aspect of comparability assessments. This approach is not limited to the bioanalytical or the clinical package alone. It also could enable predictive exploration of changes in clinical performance (e.g., through in vivo models) linked to bioanalytical changes.
Fingerprint-Like Similarity: The exact nature of our approach to extract each analytical method’s relevant information follows well established signal processing and multivariate modeling methods. Its unique aspects are centered on filtering out method artifacts, preserving each method’s contribution and scaling to attribute to each method a comparable relative importance in the final assessment of similarity.
Residual Risks: To estimate the residual uncertainty about similarity and assess robustness of the chemistry, manufacturing, and controls (CMC) filing package, several lots of both products (reference and similar) are compared. (This also happens before and after a change in the case of a comparability protocol.) A three-step evaluation proceeds in the following way (Figure 4).
First, quality attributes from all analytical domains are filtered according to their lot-to-lot variability. This ensures that only those with significant variability are included in the assessment.
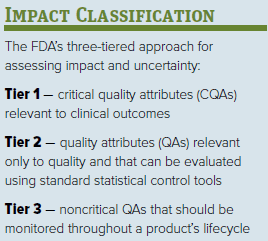 Then, a criticality assessment is performed on attributes with higher coefficients of variation (CVs). This risk-based approach allows the classification of each quality attribute (QA) as critical or noncritical by assessing impact and uncertainty. For impact classification, the FDA’s three-tiered approach can be used as detailed in the “Impact Classification” box. Uncertainty is assessed by the existence or absence of previous clinical studies, in vitro characterization of the product, and published literature.
Then, a criticality assessment is performed on attributes with higher coefficients of variation (CVs). This risk-based approach allows the classification of each quality attribute (QA) as critical or noncritical by assessing impact and uncertainty. For impact classification, the FDA’s three-tiered approach can be used as detailed in the “Impact Classification” box. Uncertainty is assessed by the existence or absence of previous clinical studies, in vitro characterization of the product, and published literature.
Finally, focusing only on Tier-1 CQAs, the purely analytical package data-driven
step conducted upfront (the fingerprint-like similarity step) can be critically assessed using a risk-based patient-focused clinical evaluation outlined in the previous steps. This establishes causal links between quality, safety, and efficacy. Our proposed approach can detect minor differences (unrelated to sampling or method artifacts) that affect a product’s activity, purity, or immunogenicity. This evaluation allows for quantitating the extent of residual uncertainty and clarifies the extent of needed similarity or comparability assessments.
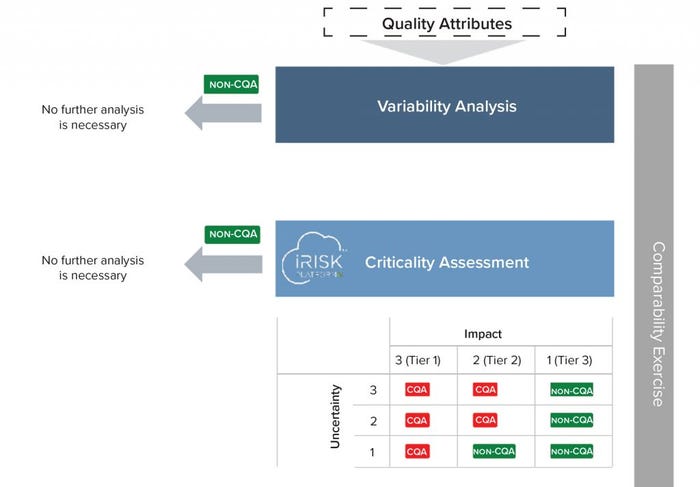
Figure 4: A stepwise and risk-based approach for biosimilarity assessments (CQA = critical quality attributes)
Mitigating Uncertainty
We have suggested a biosimilarity assessment aligned with the FDA’s totality-of-evidence concept. This multiparametric and multivariate approach facilitates a complete similarity analysis resulting in extensive comparisons between the complete domains of a biosimilar and those of its reference product. The comparability exercise is supported by a step-wise and risk-based approach that considers both the impact and uncertainty of each quality attribute on clinical outcomes. As a result, residual uncertainty is mitigated, and minor differences in terms of safety, purity, and potency can be assessed to determine whether they are clinically meaningful, establishing the link between analytical and clinical domains.
References
1 European Medicines Agency and the European Union. Biosimilars in the EU: Information Guide for Healthcare Professionals, 2017; www.ema.europa.eu/documents/leaflet/biosimilars-euinformation-guide-healthcare-professionals_en.pdf.
2 US Food and Drug Administration, CDER. Scientific Considerations in Demonstrating Biosimilarity to a Reference Product, 2015; www.fda.gov/downloads/drugs/guidances/ucm291128.pdf.
3 Menezes JC. Comparability of Biotechnology Products Subject to Changes in Manufacturing Process. Cell Culture Engineering (CCE 2016), 8<&ndash>13 May 2016, Palm Springs, CA.
4 Menezes JC. Biosimilarity and Biocomparability Assessments and Lifecycle Management. International Foundation for Process Analytical Chemistry (IFPAC 2019), 3<&ndash>6, March 2019, Bethesda, MD.
José C. Menezes, PhD, is founder and CEO of 4Tune Engineering Ltd. Anna C. Loia and Catarina S. Leitão both hold BSc and MSc degrees in bioengineering from the Technical University of Lisbon. They are MS&T specialists at 4Tune Engineering; [email protected]; www.4TuneEngineering.com.
You May Also Like






