WWW.COPPERHOUNDPICTURES.COM
Our focus on regenerative medicine this month, with its inherent personalized approach, brings to my mind aspects of “the patient experience.” Last week at BPI West in Santa Clara, CA, I was intensely moved by the performance of lymphoma survivor Toby Peach (www.tobypeach.co.uk). Our KNect365 colleagues will be asking him back this fall for the BPI Conference in Boston, and I encourage you not to miss him if you’re there. Many of us will be touched by some form of cancer in our lifetimes — whether through our own diagnoses or those of people we love — and the shared experience binds us all together.
A friend of mine recently passed away from complications related to her own battle with breast cancer decades ago. A retired nurse herself, Yvonne was a great inspiration to me when I faced cervical cancer a few years ago. Extreme radiotherapy saved her way back when, but it also devastated her lungs. For many years, she carried an oxygen concentrator in a backpack everywhere she we...
Patient cells serve as the main raw material for autologous therapies. NOVARTIS AG (WWW.NOVARTIS.COM
In the history of regenerative medicine, 2017 was a critical year. With approvals for Kymriah (tisagenlecleucel) from Novartis AG, Yescarta (axicabtagene ciloleucel) from Kite Pharma (a Gilead company), and Luxturna (voretigene neparvovec-rzyl) from Spark Therapeutics, cell and gene therapies finally made their mark on the regulatory landscape. Then in 2018, those products began both treating patients and bringing in revenues for their sponsor companies.
“Patients are being treated, and biotechnology and pharmaceutical companies are being paid for treating them,” said Anthony Davies (founder of cell and gene therapy specialist firm Dark Horse Consulting) to delegates at the Phacilitate Leaders World and World Stem Cell Summit 2019 in Miami, FL, this past January. “This is the cycle of life in drug development. We all know cell and gene therapies were approved before, but we also know that these three appro...
HTTPS://STOCK.ADOBE.COM
by Bio-Process Systems Alliance Cell and Gene Therapy Committee
The Bio-Process Systems Alliance (BPSA) was formed in 2005 as an industry-led international industry association dedicated to encouraging and accelerating the adoption of single-use manufacturing technologies used in the production of biopharmaceuticals and vaccines. Corporate members include plastic-equipment suppliers, service providers, and users in the biopharmaceutical industry who share this mission. A key focus of BPSA’s core activities is to educate its members and others through sharing of information and development of best practice guides that help suppliers, users, and regulators to safeguard the quality of drugs produced with single-use technologies (SUT).
This article is designed to provide guidance on cell and gene therapy (CGT) manufacturing, regulations, and best practices regarding implementation of single-use components. It is largely based on experience gathered from the use of these products in the...
WWW.ISTOCKPHOTO.COM
A “sea change” in the biotechnology and pharmaceutical industries is leading established players to recruit a new type of drug-development leader. Disruptive innovators such as LG Chem Life Sciences, Google, and Nestlé are challenging established life-science companies to be nimbler, more creative, and more adept at applying new and emerging technologies. To spur creativity and entrepreneurship in research and development, smaller companies and Big Pharma corporations alike are recruiting leaders from different fields both inside and outside the life sciences. They want leaders who are comfortable pushing complex boundaries, who have emotional intelligence and soft skills, and who exercise vision and risk-taking in addition to their scientific knowledge.
The new drug development leaders are skilled at negotiating codependencies between small and large companies. Start-ups and emerging companies often rely on Big Pharma for funding, robust analytics, and distribution and commercializati...
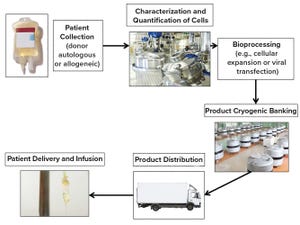
Figure 1: Lifecycle of cellular therapies
Cellular therapies can be classified by therapeutic indication, by cell types, and by whether cells are taken from and administered to the same individual (autologous) or derived from healthy donors (allogeneic). Regulatory classification of cellular therapies differentiates among minimally manipulated cells for homologous use, transplants or transfusions, and cells that are more than minimally manipulated and regulated as medicines. Medical cellular therapies must meet quality, safety, and efficacy standards to obtain marketing authorization (
1
–
8
). Such therapies can be subdivided into somatic cell, gene therapy, and tissue-engineered products. They can be manufactured from autologous or allogeneic sources and contain noncellular components (e.g., chemical or biological compounds and matrices).
Off-the-shelf allogeneic products are based on cells obtained from healthy donors and modified with gene editing to knock out functions such as immune rejection respon...
The “Single-Use Technology: Scientific and Technological Advancements” conference took place last autumn in the Wasatch Mountains of Utah. (WWW.ISTOCKPHOTO.COM)
Single-use technology (SUT) has been used increasingly both in clinical and commercial biomanufacturing (
1
). Proven major advantages include relatively low capital investment, elimination of batch-to-batch cross contamination and reuse cleaning validation efforts, flexibility in manufacturing, and shortened product lifecycles. However, some challenges and barriers to implementation remain: Consumables costs are increasing. Specific regulatory guidance is lacking, as is component interchangeability and standardization. And few if any leak-proof components/systems are available. International groups and associations focused on setting best practices and standards to ensure reliable and risk-free biomanufacturing include the American Society for Testing and Materials (ASTM), the BioPhorum Operations Group (BPOG), the Bio-Process Systems Alliance (B...
HTTPS://STOCK.ADOBE.COM
At the Phacilitate Leaders World and World Stem Cell Summit 2019, held 22–25 January, Steve Goodman (head of drug product manufacturing at bluebird bio) and Robert Di Scipio (CEO of Skyland Analytics) shared the podium to address what the product and process data-management ecosystem looks like for cell and gene therapy (CGT) development and manufacturing. A starting point for their presentation was that CGT development presents significant data challenges: capturing and analyzing product development and manufacturing data, tracking the collection, transporting and maintaining the quality of apheresis material, and analyzing these data coherently with the pertinent patient medical records. Skyland and bluebird are collaborating to ensure that bluebird has a CFR Part 11 compliant data management system that supports data transparency and analytics throughout its supply chain — as well as enabling CPV (continued process verification) and APR (annual performance reports).
Figure 1: Th...
Parenteral pharmaceuticals must be “essentially free” from visible particulate matter (
1
). In the production of biopharmaceuticals with single-use systems (SUS), biocompatibility requires controlling interactions between drug substances/products and SUS surfaces to ensure drug product quality and patient safety with regard to extractables/leachables and particulate matter. Any particulate matter stuck to fluid-contacting surfaces of process components could wash off and contaminate process fluids. Depending on system configuration, a final drug product could be at risk for particulate matter from SUS. Risk assessments take into account potential particle sources (e.g., ingredients, SUS surfaces, final containers) and particle sinks (filtration and other purification steps) throughout an entire manufacturing process. Applications of SUS downstream of final filters presents the highest risk scenario, in which direct contact between drug substance/product and SUS components occurs. For example, application...
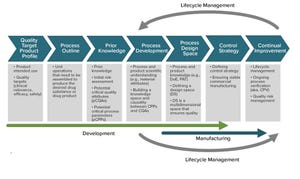
Figure 1: ICH guidelines dealing with quality as manufacturing science have focused significantly on risk (R), risk-management (RM), knowledge (K), and knowledge-management (KM). Comparisons can be made across documents through scaling of the different frequencies used (intra and inter documents, a total of 975 instances).
In their lifecycle development and manufacturing models, biotechnology products and the biopharmaceutical industry have been founded on principles originating from the pharmaceutical small-molecule industry. Such principles define clinical programs that establish risk benefits of a dosage and its delivery system on healthy individuals and patients. A company then develops a process to manufacture that product consistently over several years. Product quality attributes set through manufacturing controls are expected to ensure patient outcomes in terms of safety and efficacy and deliver the clinical performance designed, irrespective of patient-to-patient differences and variability in ma...
Talent is the lifeblood of pharmaceutical innovation. But there’s more to winning top talent than simply recognizing the value of our “human resources.” It also takes putting that value into action by giving people a workplace that inspires and even delights. Today, forward-looking industry leaders are seeing competitive advantage in rethinking workplace strategy and the role it plays in attracting and retaining talent, according to a 2018 report (
1
). Once considered to be merely a passive background for discovery, a life sciences facility can be designed to play a more meaningful role in employee collaboration, commitment, and engagement. Organizations that offer connected, sophisticated, and flexible laboratories in desirable locations — including highly attractive amenities — are feeding the human experience. And in turn, they’re attracting the brightest and best to join their ranks.
Three Ways to Keep Talent Happy
From high-tech laboratories and in-house incubators to parking-lot food trucks, Millen...


schedl_b_and_w.jpg?width=100&auto=webp&quality=80&disable=upscale)