- Sponsored Content
- Gene Therapies
- Pre-Clinical & Clinical Trials
Is the QbD Toolbox Ready for Cell and Gene Therapies? Integrating Patient Outcomes into Manufacturing Cell and Gene Therapy BioproductsIs the QbD Toolbox Ready for Cell and Gene Therapies? Integrating Patient Outcomes into Manufacturing Cell and Gene Therapy Bioproducts
Sponsored by 4Tune Engineering
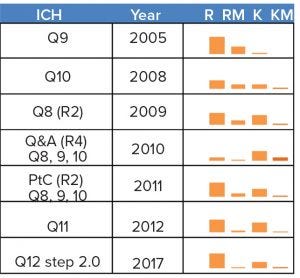
Figure 1: ICH guidelines dealing with quality as manufacturing science have focused significantly on risk (R), risk-management (RM), knowledge (K), and knowledge-management (KM). Comparisons can be made across documents through scaling of the different frequencies used (intra and inter documents, a total of 975 instances).
In their lifecycle development and manufacturing models, biotechnology products and the biopharmaceutical industry have been founded on principles originating from the pharmaceutical small-molecule industry. Such principles define clinical programs that establish risk benefits of a dosage and its delivery system on healthy individuals and patients. A company then develops a process to manufacture that product consistently over several years. Product quality attributes set through manufacturing controls are expected to ensure patient outcomes in terms of safety and efficacy and deliver the clinical performance designed, irrespective of patient-to-patient differences and variability in manufacturing operations over a product’s lifecycle.
Such a model is not optimal for biologics, and it has introduced potential risks for patients. In fact, fundamental insufficiencies and inadequacies of the pharmaceutical model exist when transported to biopharmaceutical operations. Recently, some of those potential and critical risks have started to be acknowledged and will become more apparent as more targeted therapies and new modalities are developed and introduced.
Biologics have, in general, a million times more quality attributes than small molecules; not all are clinically relevant, but the fraction of those that are is in great contrast to what is observed for small molecules.
Currently individual new biologic modalities (neither innovators nor biosimilars) do not have documented lists of all critical and clinical performance quality attributes at product launch. That is why pharmacovigilance (postmarketing) programs exist and why immunogenicity is the subject of investigation during and after commercial launch. Over the commercial lifecycle of biologic products, some CQAs will be affected by factors that are not fully known or controlled through manufacturing operations.
Therapeutic outcomes (related to efficacy and patient safety) and product specifications (CQAs) for biologics are complex, multifactorial, and extremely patient-specific.
Comprehensive, clinically relevant specifications set by adequate controls in the manufacturing processes are as yet unachieved for biopharmaceuticals.
No other aspect of a manufacturing process other than ensuring a very high degree of quality compliance at all steps provides a way to meet desired patient outcomes (efficacy) while guarding against unwanted outcomes (safety).
Although this paradigm has worked for pharmaceuticals over the past century, it is highly inefficient. Time to market is slowed, and companies repeat experimentation because metadata on previous programs may be incomplete or unclear. Investigators resort often to trial-and-error experimentation because prior knowledge is unavailable or unharvested. Lessons learned are not captured comprehensively, so portfolio management on programs using the same technology platform rarely leverage all they could from one program to the next. Troubleshooting operations over a product’s lifecycle become root-cause investigations requiring repeated research studies and experiments. And risk-based or evidence-based justification of postapproval changes is difficult to establish scientifically because the knowledge assets are either not readily available or exist in an adequate format for reuse.
Is the current model suitable for cell and gene therapy (C>) products? Can we deliver tailored products to terminally ill patients and do so effectively — right-first-time? No recalls or second attempts are possible or make any sense in these cases. There is no time for quality by design (QbD) the way it has been used for small molecules going into oral solid dosages. What will be the right risk–benefit balance that can justify speeding up through development to deliver one lot for one patient just in time? And even if a solution for C> products might be far away, why can’t the current model be streamlined? How can anyone justify that, after spending up to 50% of a patent’s 20-year lifetime and the multibillion dollars that most development programs cost, important knowledge gaps still affect operations and patient safety to the extension observed today?
Regulatory Context: Vision vs Reality
The FDA truly pioneered most of the disruptive concepts introduced over the past two decades, with its vision of “a maximally efficient, agile, flexible pharmaceutical manufacturing sector” and CGMPs for the 21st century, later further worked and adopted by other ICH regions. Other concepts followed, including process analytical technologies (PAT, 2001–2004), QbD (2004–2009), lifecycle management (2011), continuous process verification (2011), similarity assessments based on “totality of evidence” (2015), formal risk management over lifecycle, and science-based justifications of control strategies (2005–2017). All those came into specific ICH guidelines that, as a whole, establish quality as manufacturing science for pharmaceutical operations.
A closer look at the ICH guidelines, however, shows two aspects: One, they were built for small molecules, not biologics; and two, significant uncertainty exists in the proposed science-based framework (Figure 1) even for small molecules, so that risk management is still prescribed.
Handling Uncertainty — Risk Management: Biologics and especially new therapeutic modalities have hundreds or more potential critical quality attributes (pCQAs) compared with the very few specifications in making a small molecule QTPP (quality target product profile). Establishing a capable control strategy for a biologic product requires not only a gigantic effort in screening the very large pCQA space, but also accepting the residual uncertainty that always will be present over all stages of development and manufacturing.
During commercial manufacturing pharmaceutical and biopharmaceutical companies alike recall product lots, engage in corrective and preventive action (CAPA) procedures, manage a large number of postlaunch changes, and might even go for postapproval submissions to fix what was not completely addressed at a smaller, noncommercial scale earlier during development.
Every time a developer cannot measure or completely evaluate a system’s state, there will be uncertainty about that system’s behavior and how it can be best controlled. Risk-based approaches are brought in to handle such complex evaluations.
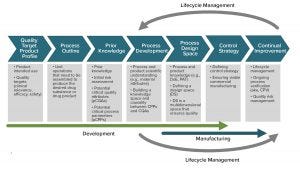
Figure 2: Nowhere in the QbD workflow can we find explicit consideration of patient-focused outcomes or differentiated patient subpopulation responses. The current QbD way to design and operate processes is therefore inadequate for patient-specific therapies or for rare diseases (almost 5% of the world population). ADAPTED FROM ISPE PQLI GUIDE, 2011, UPDATED IN PHARM. ENG 30(2) 2010: 1-6.
Designing for Patients: The QbD paradigm generally keeps patients out of the equation, using quality as a surrogate for efficacy (Figure 2). Quality target product profiles (QTPPs) used for biologics are designed for an average unspecific patient response. That works well for average clinical outcomes, with a verifiable, causal, and robust link to quality specifications. But it is an inadequate way to deliver ensured outcomes to small, differentiated patient populations.
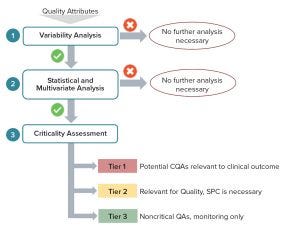
Figure 3: Critical quality attributes (CQAs) to include in a QbD workflow (Figure 2) linking clinically relevant specifications to biopharmaceutical operations and science- and risk-based justification of control strategies
However, using patient profiling and information about outcomes alone will not deliver the predictive framework required. What is needed is an effective end-to-end integration of operations over a product’s lifecycle with a true end-point on operations placed at the patient outcomes. That, and capabilities to combine knowledge across clinical programs and product portfolios, will enable sound platform approaches toward obtaining predictive and designed clinical outcomes. The type of process-modulation required to ensure the best patient–product match will be relatively small within such framework (limited to interpolations inside multipatient design- and control-spaces). Combining evidence-based and risk-based approaches to link biopharmaceutical operations end-to-end over a product/process lifecycle and to consider patient-focused specifications explicitly will have a dramatic impact on delivering predictive clinical outcomes in a sound regulatory approach (Figure 3).
This is true for demonstrating similarity and interchangeability in biologics, but it is especially more important to in the field of abbreviated filing required by accelerated patient access to new therapies. Integration in a true 4.0 sense of the main steps in biopharmaceutical operations (horizontal integration) and orthogonal lifecycle management (vertical integration) is a necessary condition, but not the only one. For biologics and new therapeutic modalities such as C>s in particular, a purely datadriven “Industry 4.0” model is insufficient. A risk-based framework capable of managing residual risks of incompletely defined systems, such as those found in biomanufacturing in general, will be required to deliver on the promise of an agile industry focused on patient outcomes.
Delivering Consistent Therapeutic Outcomes
We hope here to have provoked thoughts about shortcomings of the QbD paradigm applied to advanced new therapeutic modalities while providing suggestions about how it could be adapted to include patient outcomes. The three main components of our approach and suggestions made are
end-to-end and over lifecycle linkage of biopharmaceutical operations, from development to manufacturing, as an effective 4.0 approach to aggregating evidence and extracting insights for science-based decisions
risk-based approaches to harvest prior knowledge and manage residual uncertainties in decision-making
and the use of an explicit risk-based tiered approach to assess quality and link operations to patient outcomes.
We have worked extensively with biopharmaceutical companies across those three areas to develop commercial validated tools for each of them.
The biopharmaceutical industry is entering a new and exciting era in which practices must depart from those inherited from small-molecule pharmaceutical operations. We are confident that companies embarking on such a journey will be able to deliver consistent (and ensured by design) patient therapeutic outcomes for both existing and new biologic modalities.
José C. Menezes, PhD, is founder and CEO of 4Tune Engineering Ltd. Madalena R. Testas, MSc, Sofia T. Santos, MSc, Silvia C. Ribeiro, MSc, and Catarina S. Leitão, MSc, each holds a BSc and MSc in bioengineering from TU Lisbon; [email protected]; www.4TuneEngineering.com.
You May Also Like






