In this month’s lineup, you’ll find a common theme of balancing operating costs against company cultures and corporate philosophies. This is far from new: You can’t responsibly run a business without making such calculations!
But the value in taking a fresh look at them lies in how we are forced to dust off and reassess some assumptions (even prejudices). It is easy (and all too human) to persist with outmoded assumptions — and we cannot afford to do that given the rapid rate of change over the past century. Analyzing the repercussions and complex interrelationships of all factors that contribute to success appears to require ever greater sophistication of predictive and analytical methods.
Herein you will find another set of ROI calculations in the continuing series by Sid Jain. He presents a highly pragmatic, multifactorial approach that echoes some technical design-of- experiment reports — and you can decide whether and how to incorporate it into your company’s overall operating strategy. His analysis...
Niche-Disease: Amyloidosis
by Cheryl Scott
Amyloidosis is a family of rare diseases involving a build- up of misfolded (and thus insoluble) amyloid protein in one or more organs. The condition affects different organs in different people, involving different types of amyloid protein. It most frequently affects the heart, kidneys, liver, spleen, nervous system, and digestive tract. Severe amyloidosis can cause life-threatening organ failure, and there is no cure. Some 60 amyloid proteins have been identified, with about half of them associated with a form of the disease.
The three most common forms of this disease are amyloid light-chain (AL) amyloidosis; inflammatory amyloid A (AA); and transthyretin amyloidosis (ATTR), an inherited form caused by a mutation in the transthyretin (TTR) gene. Diagnosis typically comes later in life. In the United States, a few thousand new cases of AL amyloidosis are diagnosed each year. AA amyloidosis is the most common form in developing countries, where it can be complic...
https://bioprocessintl.com/wp-content/uploads/2015/02/022015_Langer.mp3
We recently completed an analysis of the past 30 years of industry progress in commercial-scale expression titers and bioprocessing yields.
These basic measures of biopharmaceutical manufacturing efficiency also benchmark the technological progress made in bioprocessing over recent decades. Titer and yield improvements generally indicate related bioprocessing cost savings, something most commercial-scale manufacturers work to improve. This focus on efficiency and productivity has led to constant bioprocessing improvements even for long-approved and -marketed products. Our findings indicate that although upstream titers have improved significantly, the same can’t be said for downstream yields.
This extensive review and evaluation of historical and current titer and yield data represents perhaps the only such comprehensive analysis to date. We retrieved titer and yield data for nearly 40 biopharmaceuticals (all recombinant proteins) wi...
https://bioprocessintl.com/wp-content/uploads/2015/02/022015_Jain.mp3
A company’s success is associated with its growth. An expanding company hires more employees (full-time equivalents, FTEs) to support its commercial products, to develop new products, and to provide administrative services. As it grows, an organization can become more complex and its operations less efficient. Analysis of 2013 financial data showed that revenue of US biopharmaceutical companies increases with a growing number of FTEs at a slower rate than does cost (
1
). That study assumed that all US biopharmaceutical companies have similar organizational and operational structure, regardless of their size and product characteristics (e.g., primarily biologics, primarily small molecules, or diversified product portfolio). Relationships of revenue and cost with number of FTEs for the biopharmaceutical industry overall were determined as in Equations 1 and 2.
Equations
Return on investment (ROI) is calculated as revenue/cost (Equation 3...
https://bioprocessintl.com/wp-content/uploads/2015/02/022015_SpecRpt.mp3
by Antoinette Lagerwij, Sudeep Suman, Jamie Hintlian, and Kevin Chen
Since the business of making vaccines became a commercial proposition, profitability has often been elusive. The economics are difficult: Costs of development and production, already high, are rising. Profit margins historically have been lower than those of other pharmaceutical products, in part because of the complexities of manufacturing and distributing vaccines as well as their stringent safety, testing, and quality requirements. And scaling-up of immunization programs along with the introduction of new vaccines have put a significant strain on decades-old logistics and delivery systems. All that has been too much for many major manufacturers, especially in the United States. Several have left the market, leaving the vast majority of the world’s production (90%) and two-thirds of research and development (R&D) to companies in Europe (
1
).
But the economics of ...
https://bioprocessintl.com/wp-content/uploads/2015/02/022015_AntibodyFragment.mp3
Antibody fragments are potent active drug substances (
1–4
). Because they lack glycosylation, they can be produced using different biological expression systems, including yeast and microbial systems as well as mammalian cells. These molecules are interesting as biopharmaceuticals because they are smaller than full-size antibodies and therefore may penetrate better into different tissues.
Antibody fragments are cleared faster in biological systems because they lack the Fc antibody structural region (
4
). However, fragments may be conjugated to increase their size for improved pharmacokinetics, for example using polyethylene glycol (PEG). Certolizumab pegol, a PEGylated IgG Fab fragment, has been on the market since 2008 (
4
), and many other such products are in clinical trials. Engineered antibody fragments such as scFv, scFab, diabodies, and divalent fragments (DiFabodies) also are under investigation (
1–3
). Fragments...
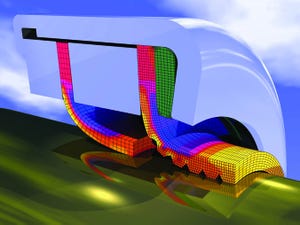
Finite-element modeling is an attractive alternative to physical testing for predicting seal life, particularly when aging poses major concerns and seal replacement is expensive. For years, seal manufacturers and users alike have searched for a reliable method for predicting how long seals will last in service.
The FEA modeling process simulates seal reliability in real-world scenarios.
Past methods for evaluating an elastomer’s potential as a static or dynamic seal use American Society for Testing and Materials (ASTM) or other standard immersion tests. These tests involve submerging a material in a test fluid for a specific time. Technicians then compare physical properties (e.g., hardness and ultimate tensile strength) before and after immersion and make a judgment about the material’s suitability as a seal.
Immersion testing certainly plays a role in screening potential materials. Of course, a knowledgeable engineer would not choose a material that severely deteriorated in an immersion test. But some p...
To date, the majority of recombinant monoclonal antibodies (MAbs) have been produced by mammalian cells. During such production processes, the potential risk of entrained viruses must be critically considered (
1
). Contamination can arise from animal cell lines or from adventitious viruses introduced during manufacturing. To ensure the viral safety of biotechnology products, companies can take four complementary approaches (
2
,
3
):
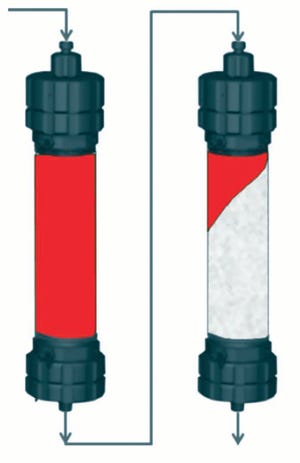
In most antibody-purification processes, the first viral clearance step is a low-pH inactivation (pH <4) conducted after protein A affinity capture chromatography. Precipitation of impurities often occurs in this low- pH treatment and subsequent conditioning, and filtration is required afterward before further processing in chromatographic polishing steps. Depth filters are commonly used (Figure 1).
Figure 1: Antibody purification process with adsorptive depth filtration as a viral clearance step orthogonal to low-pH inactivation and virus filtration; the additional virus r...
Protein A affinity chromatography offers efficient monoclonal antibody (MAb) purification and is used extensively in large-scale MAb production. As is the case with most chromatography media, protein A resins often have some degree of nonspecific binding, which causes host-cell proteins (HCPs) to coelute with a MAb. To reduce nonspecific binding interactions, an intermediate wash step can be performed before product elution. Doing so can improve product purity, extend column lifetime, and potentially eliminate a subsequent polishing step. For large- scale purification processes, it can be worthwhile to optimize the wash and elution conditions to maximize yield. Intermediate wash conditions are often dependent on specific feeds with specific resins. This study showcases two specific CHO feeds with Eshmuno A resin.
A wide range of intermediate wash conditions for protein A chromatography have been described elsewhere (
1–5
). Those include different pH and salt conditions, as well as the presence of various...
Accelerating the commercialization of promising new cancer treatments relies on ensuring that patients — individually and collectively — are actively involved throughout research and drug development. This was the consensus of leading scientists, advocates, and government officials meeting in Washington, DC, at the first national policy forum convened by the Cancer Innovation Coalition (CIC). A collaboration of cancer stakeholder organizations and others working through a national campaign called Project Innovation — which is spearheaded by National Patient Advocate Foundation (NPAF) — the CIC aims to elevate cancer innovation to a national priority. To move cancer discovery forward, experts are calling for greater use of innovative clinical study designs, creating clinical trial networks and sharing data, and streamlining institutional and regulatory obstacles that hinder scientific discovery and drug development.
Innovation is the key to progress, but it is not happening fast enough. That is why we need...