The real word
twitterpated
(meaning infatuated or obsessed, in a state of nervous excitement) apparently was coined in Disney’s
Bambi
movie. Use of the word has increased over the past five or six years, achieving a new relevance. Now it can mean Twitter-headed, and I am finally beginning to catch on to the role that tweeting plays, for good and ill. As expected from a Hollywood word, it focuses our attention on the ephemeral, the superficial glitz of a person or event. And that element bothered me at first: Where is the substance, the interpretation — not to mention the grammar?!
Whether a person takes to social media as a duck to water or has to learn new ways to swim (or just stays out of the water entirely) is a matter of personal learning styles more than generational differences. These programs are tools just like MS Word and Adobe Acrobat, and receptiveness to learning them is a choice. Besides, we do need the occasional reminder to “look here!” and see those “really cool, wow!” things. I see i...
Introducing New Editorial Advisor
Jason Condon
is a senior CMC project manager at Vaccinex, Inc., in Rochester, NY as well as an assistant adjunct professor in biomedical engineering at the University of Rochester. He has held roles in biopharmaceutical manufacturing and process development at Bristol Myers Squibb and Janssen R&D. His expertise in cell culture and current good manufacturing practice (CGMP) manufacturing has contributed to developing manufacturing processes for monoclonal antibody (MAb), cell therapy, and vaccine production throughout his 13-year career in the industry. A significant portion of that work involved continuous (perfusion) bioreactors and hollow-fiber filter cell-retention devices.
Condon is responsible for the clinical supply chain at Vaccinex, where he oversees drug-substance and drug-product manufacturing and testing along with packaging, labeling, and distribution of finished products. He has written or contributed to the CMC section of numerous investigational new drug (...
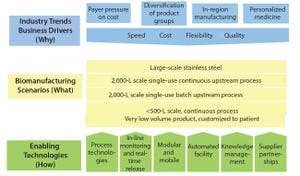
Figure 1: Roadmap development and structure
For a complex biopharmaceutical industry, setting out to forecast future technologies must involve considering how such technologies will be used. In the
first article
(
1
), I discussed why there was a need to develop a technology roadmap for the biopharmaceutical industry and the trends shaping its future: namely, the introduction of new product classes, the continued growth of the biopharmaceutical market, pressure to reduce costs, and uncertainty in approval and sales of new products. Herein I discuss the technology roadmap’s objective, the approach taken to develop it, business driver metrics, and (most important) the scenarios that consider how technology might be used in the future. I will also introduce the six teams looking at key technologies and enablers.
Background and Objective
Over 150 individuals participated in designing and constructing the technology roadmap. They include representatives from more than 17 biopharmaceutical manufacturers, 12 s...
www.photos.com
Biotechnology has enabled commercialization of protein-based drugs including insulin, growth factors, blood factors, and antibodies. Production and purification of such biologic products require different buffers for pH control and stabilization of reactions in different steps during biomanufacture.
These processes include cell culture production (the “upstream” phase), purification (the “downstream” phase), and a final phase in which excipients are introduced to the drug substance to create a drug product (“formulation and storage”). In upstream processes, buffers are primarily used for their capacity to help maintain culture pH within a specific range, optimizing conditions for cell growth and expansion, promoting production of a desired protein, and maintaining that protein’s functional characteristics during primary recovery before purification.
In downstream processing, buffers maintain defined purification conditions, control a protein’s ionization state as necessary for column chroma...
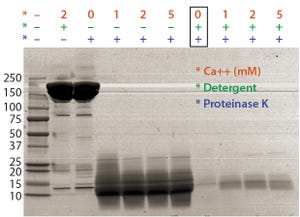
Figure 1: Optimal proteinase K digestion in the presence of detergent and/or calcium ion
Regulatory guidelines that cover the development of biopharmaceutical products require testing of host-cell deoxyribonucleic acid (DNA) impurities. Real-time polymerase chain reaction (PCR) has become a popular technology for DNA quantitation and monitoring of process impurities associated with biomanufacturing. One critical challenge associated with host-cell DNA impurity testing is that recombinant proteins (e.g., monoclonal antibodies, MAbs) and their corresponding buffer components often interfere with DNA quantitation in real-time PCR reactions (
1
,
2
). Some sample types do not require a full extraction and can be quantitated by a real-time PCR directly. But because of the complexity of bioprocess sample matrices — from upstream to downstream processes — a full extraction typically must be executed.
We previously developed and validated a sodium iodide-based extraction method for removal of matrix interference...
WWW.ISTOCK.COM
As stated in ICH Q6B,
specifications
are critical quality standards that are both proposed and justified by drug product manufacturers. Xiaoyu et al. provide information on several statistically based strategies to establish specification acceptance criteria (SAC) (
1
). Here we address an alternative approach to relate proposed SAC for quantitative data to relevant lot history. In particular, proposed SAC can be derived in part by using calculated limits for which the lower bound of an approximate 95% confidence interval for the process performance index (
P
pk
) is ≥1 (
2
).
This “minimum
P
pk
” strategy uses a statistical criterion based on manufacturing experience alone. The minimum
P
pk
approach is not intended to be the sole consideration for the determination of SAC. Other factors such as product stability, analytical procedures, and nonstatistical justifications from a scientific and regulatory perspective should be considered in determining appropriate SAC.
Process Capability ...
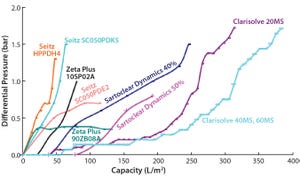
Figure 1: Harvest clarification options
Steadily increasing demand for biopharmaceutical drugs has led the industry to examine its manufacturing scales while pressuring research and development groups to produce high-yielding clones and processes. Improved media, feed supplements, bioreactor designs, and control of process parameters have helped biomanufacturers achieve multifold increases in volumetric productivity from production bioreactors.
However, cell culture processes are significantly affected by their bioreactor’s ability to support cells at higher densities and sustain cultures at lower viabilities. With the implementation of a number of new approaches, cell densities have been increased from 5–7 × 10
6
cells/mL to >25 × 10
6
cells/mL. Such increased densities — and improved specific productivity of the modern clones — has increased productivity as well, with expression titers rising from 1.0 g/L to 5–8 g/L in fed-batch cell culture processes. Moreover, concentrated fed-batch and perfusion ce...
During the Biotech Week in Boston this past October, I had a chance to talk with David DiGiusto (Stanford University) about his work toward advancing bioprocessing and cell therapy development. I asked him to comment on points from his keynote presentation about how academic research groups can sustainably cycle assets into the biopharmaceutical pipeline. University research departments have long made innovative technologies available for commercial licensing. But in the excerpt below, he details ways in which such groups are further supporting and driving clinical evaluations, derisking potentially life-saving therapeutic candidates, and thereby shortening the time to commercial launch. (You can find the video of our full conversation at
http://www.youtube.com/watch?v=5rpCzljZ6tI
.)
An Engine for Innovation
Stanford University is an engine for innovation centered in the Silicon Valley. With the large number of investigators at Stanford, we have a vast pipeline of cell and gene therapy applications and p...
In BPI’s 5 October 2016 webcast, Paul Jorjorian (director of global technology transfer at Patheon) discussed his top five considerations for companies outsourcing to a biomanufacturing partner.
Jorjorian’s Presentation
Selecting a contract and development manufacturing organization (CDMO) is an important decision for many biopharmaceutical companies. Beyond time and cost, here are some other criteria to consider.
The number-one issue is
quality.
Is your company’s definition of quality the same as a CDMOs — and if not, how do those definitions differ? Here are three main discussion points: understanding how a CDMO addresses deviations; creating a quality agreement; and determining who is responsible for making, reviewing, and approving required documents.
The second consideration is
technical expertise
. Does a CDMO’s technical expertise fit your needs? Examples include experience with the different phases of drug development, process characterization studies, recombinant or fusion proteins, monoclonal...