January-February 2019
WWW.LIFETOUCH.COM
Greetings from the BPI editorial staff — we wish you a healthy and prosperous 2019! Beginning with a combined January–February issue somehow makes the year seem to be speeding by right from the start. We are immersed now in the first radical redesign of our magazine since the publication’s founding in 2003. Some aspects proceed smoothly; others, not so much. You see with this issue a new cover design that ties us in with our KNect365 event brands. You’ll see similar changes on our eBooks and featured reports as well.
But then . . . we thought we knew enough about fonts and their licensing. As fun as they are to play with, and as much as some can help publications to look and feel “modern,” a number of fonts are less technically viable for print than others. Late last year, our technical editor efficiently revised all our templates based on a short list of official font choices from a corporate rebranding effort. We quickly started using them with editorial glee — only to find out a week ...
Launched in June 2018, the
BioProcess Insider
digital information portal delivers the latest financial and business news and expert insider views influencing the commercialization of biopharmaceuticals. Here are just a few recent stories edited for our space limitations in print. For more discussion and in-depth analysis, check out the website at www.bioprocessinsider.com and sign up for the twice-weekly newsletter.
AstraZeneca Closing Two Colorado Sites
Anglo-Swedish pharmaceutical giant AstraZeneca is ending operations at its facilities in Boulder and Longmont, CO, less than four years after acquiring them from Amgen. The decision to shutter both sites was made as part of a biomanufacturing consolidation strategy, a spokesperson told
BioProcess Insider
.
“AstraZeneca has made the decision to consolidate the biologics manufacturing network in one large-scale drug-substance facility: our site currently operating in Frederick, MD. Neither Boulder nor Longmont is currently licensed for commercial operati...
WWW.ISTOCKPHOTO.COM
The biopharmaceutical industry has seen strong growth over the past 15 years, with a compounded annual growth rate (CAGR) of 6.7%. However, if you look at that growth in five-year intervals, it becomes apparent that the rates have begun to decline in many countries. Companies have withdrawn somewhat and put initiatives into place to curb further increases in the cost of drugs. The CAGR fell from 8.7% for the period of 2002–2006 to only 3.1% for 2012–2016.
In our yearly cross-industry global pricing and sales study (
1
), 94% of managers from the biopharmaceutical industry reported that they have been facing increasing price pressures. The industry’s research and development (R&D) spending reached an all-time high of US$173 billion in 2017. So there should be no shortage of new products; however, only about half of all new product launches meet their sponsor companies’ profit expectations. In other words, R&D money is being spent ineffectively, and companies need to rethink their busine...
HTTPS://STOCK.ADOBE.COM
Contract manufacturing organizations (CMOs) are a core part of the biopharmaceutical industry, with commercial manufacturing making up about a third of marketed products. Here we examine worldwide CMO biopharmaceutical manufacturing (bioprocessing) capacity, concentrating on “mainstream” CMOs — defined as those providing primarily mammalian cell culture or microbial fermentation services for manufacture at any scale of proteins and antibodies. This analysis follows our recent article examining worldwide bioprocessing capacity status and trends (
1
).
We highlight CMO-related results from BioPlan Associates’ 15th annual
Report and Survey of Biopharmaceutical Manufacturing
, which includes responses from 222 biopharmaceutical developers/ manufacturers and 130 bioprocessing suppliers or vendors. In our 2018 survey, more than half (56%) of bioprocessing professionals surveyed reported that their facilities outsource at least some bioprocessing, with 19% outsourcing more than half of t...
ADOBE STOCK (HTPPS://STOCK.ADOBE.COM)
Immunotherapy seeks to harness the power of our human immune system to fight disease. In this rapidly evolving field, collaboration among different stakeholders is essential to bringing new treatments to market. Patient advocacy groups, researchers, hospitals, manufacturers, and government entities all are working together to translate promising new research into life-saving products. Types of immunotherapy include monoclonal antibodies (MAbs) and antibody derivatives, checkpoint inhibitors (immune-modulating proteins), cancer vaccines, T-cell therapies, and cytokines — so the approach involves a range of product modalities: proteins, gene therapies, and cell therapies. Thus, their manufacturing issues run the gamut of bioprocessing. Product development involves specialized clinical trials and efficacy test methods, however.
With the complex nature of human immune systems and cancer biology, novel collaborations are needed to turn exciting research into viable treatme...
WWW.LONZA.COM
The biopharmaceutical industry is under pressure to generate value for shareholders and find innovative therapies while driving down development and manufacturing costs. As the industry considers more flexible and scalable production solutions for patients, new therapies will continue to be developed faster than ever before. These products will need to rely on robust and reliable manufacturing solutions. To deliver on that challenge, streamlining manufacturing processes will be necessary so that many lots of drug substances can be manufactured, stored, and/or processed rapidly and safely. That allows for faster turnaround times between products.
Single-use freeze systems are a sound investment in current unit operations and support future capital returns capable of handling rapidly changing bioprocessing requirements. Single-use technologies (SUTs) have been implemented mainly in upstream biomanufacturing but also some downstream processing and drug-product handling operations (
1
,
2
). SU...
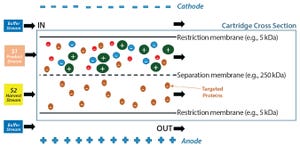
Figure 1: PRIME separation technology uses an electric field and polyacrylamide membranes with different pore sizes to isolate proteins of interest.
Human plasma is a complex mixture of biomolecules such as serum albumin, immunoglobulins, coagulation factors, and others (
1
). These important protein biomolecules often are present at low levels or lacking in affected patients with certain life-threatening conditions. To extract those valuable proteins, a number of purification methods have been developed over time. Plasma fractionation can be traced back to the middle of the 20th century, when Edwin Cohn of Harvard University developed the first industrial process to purify proteins from crude human plasma (
2
). The Cohn fractionation method still is widely used today, as is a combination of several column chromatography separations and orthogonal viral inactivation or removal methods, to produce high-quality plasma-derived therapeutics (
3
).
The Cohn fractionation method uses a protein precipitation ap...
SARTORIUS STEDIM BIOTECH (WWW.SARTORIUS.COM)
In the past 20 years, novel therapeutics have become a major segment of biopharmaceutical research and development, particularly for immune disorders and cancer. Progress in gene therapies could bring cures for once deadly and debilitating genetic disorders such as hemophilia or muscular dystrophy. Biologic drug products offer potential treatments that have not been possible with traditional (chemistry-based) approaches. But such products also are more difficult to produce cost effectively at an industrial scale because of the intricacies associated with biological host agents. Biopharmaceuticals are associated with complex biological machineries, sensitivity to heat and shear stresses, difficult characterization, and challenging identification of naturally occurring and process-related molecular variants (
1
).
The US Food and Drug Administration (FDA) defines
biologic drugs
as those “isolated from a variety of natural sources – human, animal, and microorgan...
Host cell proteins (HCPs) originate from host organisms that are used to produce biopharmaceutical products. They are in-process contaminants that must be minimized during downstream process operations. According to regulatory agencies, the maximum permitted level of total HCP in a biopharmaceutical product is 100 ng per mg (100 ppm) (
1
). HCPs can decrease drug efficacy and pose a risk to patient safety because they can bring on undesirable immune responses. Thus, HCPs are a critical quality attribute that should be minimized and closely monitored according to US (21 CFR 610.13, USP <1132>), European (ICH Q6B, EMA 3AB1A), and other regulatory guidelines (
2
–
5
).
Historically, analysis of HCPs has been performed using enzyme-linked immunosorbent assay (ELISA) tests based on polyclonal antibodies (generated to the whole host cell proteome). This approach enables detection and quantitation of HCPs as a population. Good reproducibility, sensitivity, and high throughput are some advantages of using ELISA m...
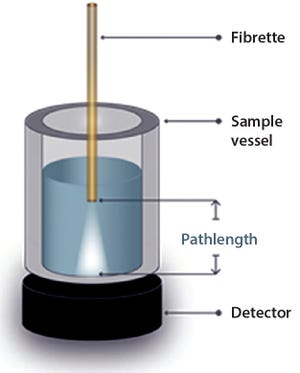
Figure 1: Sample configuration
The biopharmaceutical industry’s need for rapid, accurate concentration measurements of protein-containing products is critical. The protein-concentration assay measures ultraviolet absorption at 280 nm (A
280
) and usually is performed both as an in-process test and for product-release testing. The SoloVPE system can analyze samples across a wide range of target concentrations without the need for labor-intensive and error-prone dilutions. Slope Spectroscopy methods provide companies with a universal platform for determining protein concentration for all in-process, clinical, and commercial methods.
During in-process UV testing, turn-around time is as important as accuracy as analysts must wait for results before they can continue processing. Traditional methodologies relying on fixed-pathlength UV spectroscopy can require several hours of stagnant time because of the need for careful sample handling, preparation, and (in particular) dilutions needed for bringing samples in...
Single use in bioprocessing has changed significantly in recent years. To find out how a leading supplier to the biopharmaceutical industry is redefining its technology to align with new market challenges, science writer, Sue Pearson, had the opportunity to interview Stefan Schlack, Head of Bioprocess Marketing, and Jean-Marc Cappia, Head of Segment Marketing Vaccines, both at Sartorius Stedim Biotech (SSB) in Goettingen, Germany.
Redefining Single Use
Pearson:
Sartorius Stedim Biotech is a recognized leader in single-use technology, and you’ve recently introduced a campaign focusing on automation, biocompatibility, integrity and the supply chain; why these areas?
Schlack:
These are the main gaps, particularly with commercial-scale single-use manufacturing. For example, regarding biocompatibility, extractables and leachables can cause problems, such as those with cell growth. If just one parameter changes, either in the resin formulation or the manufacturing process, this might alter the extractables an...
An “Ask the Expert” webinar on 27 November 2018 featured Ray Marzouk, vice president of quality at Avid Bioservices, a biologics contract development and manufacturing organization (CDMO) in Orange County, CA. With six successful preapproval inspections behind it, Avid prides itself on a 13-year industry-leading regulatory track record.
Marzouk’s Presentation
Preparing for the Inspection:
Implement a standard operating procedure (SOP) for inspections that includes instructions for the receptionist, host, scribe, subject matter experts (SMEs), and document finders. Prepare SMEs for interacting with investigators. Ensure sufficient back-room support for responding to document requests on time. Perform an unannounced mock inspection to evaluate how your people handle it.
Establish a strong quality management system. No matter how well versed a company is in conducting inspections, compliance with established quality systems throughout the year ultimately determines their outcomes. Routine internal audits ca...
On 29 November 2018, Matthew Smonskey (senior scientist in the Gibco Custom Media Services team at Thermo Fisher Scientific) presented an “Ask the Expert” webinar on resources available to help developers identify media limitations and options for designing custom media formulations that maximize protein yield.
Smonskey’s Presentation
Gibco’s goal is to solve its clients’ bioproduction problems: e.g., creating a chemically defined medium, increasing end-point titer, modifying protein quality attributes, and so on. Designing custom media using spent-media analysis might solve some such problems.
Some cultures might not perform up to expectation. All immortalized cell lines experience genetic and epigenetic drift throughout their periods of use. Evolutionary pressure leads to hundreds of thousands of small mutations and larger structural variances, many biologically active in the cells. A final cell line could be very different from the starting cell line it came from, with unique nutritional requirements.
In an Ask-the-Expert webinar on 4 December 2018, Jason Chiu (product marketing scientist at JSR Life Sciences) introduced the Chromassette chromatography cassette platform. This stackable, modular technology and resin-support device presents a productive and disposable process that can scale to any volume using any resin. An internal scaffold provides a uniquely supported resin bed, and the system is fully interchangeable with conventional column formats.
Chiu’s Presentation
The Chromassette platform offers linear scalability, superior pressure-flow properties, high-productivity separations, and rapid and convenient turnover. The technology comes as self-contained individual cassettes with 4-mL volume and 6-cm bed height or 13 mL and 20-cm bed height. JSR Life Sciences is working on a 1-L cassette with a 20-cm bed height.
“Wall effect” is a well-known phenomenon in conventional chromatography columns. As column diameters widen, resin support weakens because the wall effect decreases. However, this is not ...
Precision medicine has long been a tantalizing goal for the pharmaceutical and healthcare industries. Fields such as oncology and rare diseases have benefited greatly from implementation of genome-guided care. Drug developers likewise have made strides with biomarker-aided discovery programs and stratification for clinical trials. Current indications suggest that autoimmune diseases will be the next major focus of personalized medicine.
The goal of precision medicine often is described as “getting the right drug to the right patient at the right time.” Through this model, patients would enjoy better outcomes and fewer side effects than in the traditional “blockbuster medicine” model. In practice, the biopharmaceutical industry increasingly has taken significant steps toward this goal. As early success stories in precision medicine, targeted cancer therapies have shown remarkable success in the small proportion of patients for whom they are appropriate. Proponents of precision medicine often do not mention...

schedl_b_and_w.jpg?width=100&auto=webp&quality=80&disable=upscale)