January-February 2020
WWW.COPPERHOUNDPICTURES.COM
I’ve been enjoying the inevitable social-media debate over whether we’re starting a new decade now or next year. Editors adore that sort of thing — but as much as I love accuracy, I must admit that I’m in the “now” camp. Welcome to the ’20s, anyway, whether or not you think that’s equivalent to “the second decade of the new millennium.” We humans love patterns and milestones, so watching the odometer tick over always brings about a sense of nostalgia and taking stock.
For the biopharmaceutical industry, the 2000s were all about industry maturity and the realizations that come with it. Emerging single-use technologies were all the rage, and companies were trying to figure out how the quality by design (QbD) paradigm was going to change how they work. Biosimilars were an imminent threat (or opportunity, depending on your point of view), and manufacturing capacity questions lingered from the turn of the century onward. We saw a lot of hype about personalized medicine, but little r...
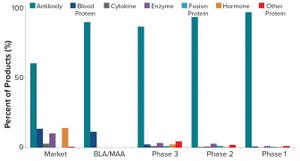
Figure 1: Distribution of mammalian-cell–based products by product type and phase of development (BLA = biologics license application, MAA = marketing authorization application)
Since the 1986 approval of the first recombinant therapeutic antibody, OKT3, biopharmaceuticals have become a large percentage of overall pharmaceutical company revenue. In 2018, sales of the top five selling recombinant proteins — Humira (adalimumab, AbbVie), Keytruda (pembrolizumab, Merck), Herceptin (trastuzumab, Genentech), Enbrel (etanercept, Amgen), and Avastin (bevacizumab, Genentech), all antibodies — totaled over US$48 billion. The compound annual growth rate (CAGR) for antibodies revenue was about 20% from 2004 to 2014. Those products include naked monoclonal antibodies (MAbs), Fc-fusion proteins, antibody fragments, bispecific antibodies, antibody conjugates, and other antibody-related products. However, that CAGR has slowed to the midteens because of the maturation of many biopharmaceutical products and emerging altern...
For many years the pharmaceutical industry was dominated by small (usually synthetic) molecules, mixed with a number of nonactive materials and encapsulated or (in the really old days) rolled into pills or pressed into tablets. Although synthesizing the active pharmaceutical ingredients (APIs), formulating the dosage forms, and analyzing the materials at every stage of a product life cycle were not always trivial activities, they were relatively straightforward. Most of the tools needed for analyzing/controlling each step of the manufacturing process were prevalent in laboratories across the world. Because early commercial production tools were very slow by today’s standards, in-process tests did not need to be fast or sophisticated. Indeed, the vast majority of early solid-dosage forms were “immediate-release” tablets or capsules that depended on gelatin-solubility for API release. Later, time-release dosage forms were subjected to the same in-process tests as immediate release forms: e.g., hardness, fri...
https://stock.adobe.com
Bioassays help drug developers determine the biological activity (potency) of their products, which has been a biopharmaceutical critical quality attribute (CQA) since long before that concept had a name. Because of their complex nature, bioassays are among of the most challenging experiments to perform reliably with dependably accurate results. Consistent assay performance requires a controlled environment and qualified reagents; skilled analysts who understand cell physiology, regulatory requirements, and the latest techniques; and assay protocols that are intelligently developed, characterized, and validated.
Statistics and risk management are increasingly important in the evolving field of biopharmaceutical analysis. Bioassay scientists are finding risk-assessment tools and life-cycle approaches to be a great help in organizing their work and putting results into context. Statistics are increasingly important to designing and optimizing biological assay methods. And new technol...
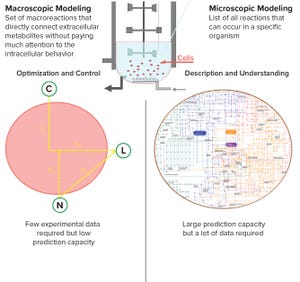
Figure 1: Systems biology tools can facilitate upstream research activities (bioreactor imagery: https://commons.wikimedia.org/wiki/User:Rocket000/ SVGs/Chemistry#/media/File:Bioreactor_principle.svg).
High-throughput technologies have transformed the biotechnology industry. The amount of data they generate is at least a hundred times higher now than it was two decades ago, primarily because of the rise of “-omic” technologies. As in many other industries, the biopharmaceutical sector entered the era of big data the day that high-throughput analytics were routinely implemented in experimental research.
Big data
refers to “datasets with sizes beyond the ability of commonly used software tools to capture, curate, manage, and process data within a tolerable elapsed time” (
1
). Technically, scientists from the field agree that big data is defined by specific attributes, the so-called 4V model: volume (scale of data), variety (different forms of data), velocity (analysis of streaming data), and veracity (unc...
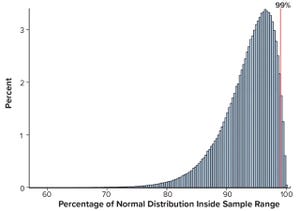
Figure 1: Percentage of normal distribution covered by the sample range,
n
= 30 with ACF(1) = 0
Calculations, including statistical tolerance intervals, can assist in the development and revision of specification acceptance criteria. Manufacturing results for attributes of a biopharmaceutical product can be positively autocorrelated. The sample standard deviation — calculated from limited, positively autocorrelated data — tends to underestimate the long-term process standard deviation (
1
). In this article, simulated data are used to assess the relative performance of statistical tolerance intervals, intervals calculated using the minimum process performance index P
pk
approach, and the sample range.
Prevalence of Positively Autocorrelated Data
The estimate of process standard deviation (σ) calculated from a limited number of batches (
n
) can be markedly underestimated when data are positively autocorrelated. The presence of positive autocorrelation (values that are closely related to each other in s...
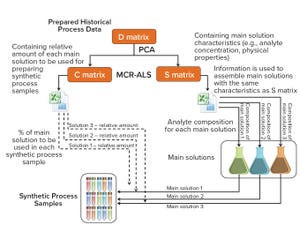
Figure 1: Method and system for preparing synthetic multicomponent biotechnological samples according to established scale-independent multiattribute monitoring calibrations; PCA = principal component analysis, MCR-ALS = multivariate curve resolution–alternating least squares (
1
)
Well-established process analytical technology (PAT) strategies, such as those based on spectroscopy, bring with them several challenges related to the nature of those tools themselves (
1–3
). Such tools are multiparametric by design — in the sense that most spectroscopies capture multiple attributes sometimes different in nature (e.g., near-infrared, NIR, captures chemical and physical attributes simultaneously). Often a reference method is required; at other times, indirect calibrations are based on the correlation of one culture attribute with another for which a chosen PAT tool lacks adequate selectivity or sensitivity. Finally, building monitoring calibrations during process development is extremely difficult when conditi...
Geert Van Raemdonck
(global field support expert at PharmaFluidics) and
Koen Sandra
(scientific director of the Research Institution for Chromatography, RIC) teamed up for a 10 October 2019 “Ask the Expert” webinar to introduce micro Pillar Array Column (μPAC™) technology for liquid chromatography–mass spectrometry (LC–MS) for host-cell protein (HCP) detection. Van Raemdonck explained that μPAC technology approaches chromatography differently than does packed-bed technology. Microfluidic channels with arrays of free-standing pillars are etched lithographically into a silicon wafer. The resulting permeability and low “on-column” dispersion provided by the perfect order of the separation bed makes μPAC-based chromatography truly unique.
Van Raemdonck’s Presentation
Current protein research relies on packed-bed LC columns of 50–75 cm coupled to high-resolution mass spectrometers to analyze protein samples from tissues, body fluids, and cell lysates. The stationary phase of a conventional column consists o...
In a 31 October 2019 “Ask the Expert” presentation,
Nicole Wakes
(group leader of Abzena’s cell-line development team) observed that drug sponsors often outsource their early upstream activities to a few different contract research organizations (CROs). But that strategy can thwart short timelines and introduce regulatory and financial risks. Wakes described Abzena’s upstream approach, illustrating how partnering with a single, multicompetent CRO from cell line construction through manufacture can streamline workflows. Integrating cell line development and manufacturing in this way promises to ease process transitions, reduce the incidence of process variability, and prevent resource duplication.
Wakes’s Presentation
Sponsors often need a few third parties to get through upstream activities. In a typical workflow, one vendor handles research cell bank (RCB) development, then passing clone candidates to another for expression stability assessment. Only after that does the original vendor — or another — s...
Biopharmaceutical companies need to make critical chemistry, manufacturing, and controls (CMC) decisions during clinical development of recombinant protein biologics and advanced therapies. In a 17 December 2019 “Ask the Expert” webinar,
Nigel Shipston
(director of program design at FUJIFILM Diosynth Biotechnologies, FDB) reviewed key aspects of selecting and working with a contract development and manufacturing organization (CDMO). He also highlighted important factors that should be considered during early stages of process development.
Shipston’s Presentation
The sheer magnitude of investment required to assemble a technically skilled workforce, procure increasingly sophisticated but expensive equipment, and maintain good manufacturing practice (GMP)-compliant facilities — together with large portfolios and increasing competition — is compelling even the largest biopharmaceutical companies to select suitable CDMO partners. With careful selection, a strong partnership can be sustained through an entir...
In biopharmaceutical manufacturing, cell culture media supply critical nutrients and maintain pH and osmolality to optimize protein product yield. Because media composition and condition have a strong effect on final biologic product quality and production, biopharmaceutical companies monitor media for lot-to-lot variability. Stability testing for degradation due to light exposure, temperature changes, or shelf-life/time is possible with rapid spectroscopic methods. In an 8 October 2019 “Ask the Expert” webinar,
O. Dean Stuart
(product manager at Thermo Fisher Scientific) explained how TruScan RM handheld Raman analyzers with TruTools onboard chemometric software can facilitate media characterization for improved control of process specifications. With those tools, users can perform detailed media assessments quickly at critical bioprocessing stages.
Stewart’s Presentation
Raman spectroscopy can enhance biologic drug quality by generating valuable data about cell culture media quality. TruScan RM device...
Image: iStock/Grafner
Animal studies can be poor predictors of human drug response. Species-specificity of organs is a concern especially for the heart. Many drugs that enter clinical trials will fail ultimately because of unexpected cardiotoxicity. Drug developers would love to mitigate such risks through in vitro human cardiac testing, but human heart biopsy materials and donor organs do not survive well in a laboratory setting.
Breakthroughs with human stem cells offer an alternative. It is now possible to take a simple skin biopsy or blood draw, then isolate and expand specific cells, then genetically reprogram them into induced pluripotent stem cells (iPSCs). Those subsequently can differentiate into any cell type, such as heart or liver cells. Human iPSC-derived cardiomyocytes can thrive in long-term culture, and their nearly unlimited supply enables bioengineering efforts aimed at creating in vitro models of the human heart.
Recent developments in tissue engineering now provide a wide range of huma...
Armin Hauk and Alexander Trappe
Extractables and leachables (E&Ls) must be addressed in material and process validation programs. Extractables are compounds that can be extracted from a material in the presence of solvents with varying polarity under extreme conditions. Materials manufacturers should make extractables guides available to end users. Leachables are compounds that migrate from a material in the presence of an actual formulation under normal process operating conditions. Extractables information can be helpful as a basis for evaluation of potential process-equipment–related leachables (PERLs)testing.
However, extractables data and potential leachables must be correlated carefully. After over 20 years of E&L testing, the industry has found very few leachables–drug interactions. With a fully elucidated extractables profile, it is possible to extrapolate leachbales. In most single-use systems (SUSs), only a low number of PERLs become true leachbales and only at very low concentrations.
In contai...