WWW.LIFETOUCH.COM
One thing that differentiates Baby Boomers, GenXers, Millennials, and Generation Y is their communication preferences. Who is most likely to answer a call or email, default to texting, or use a given social-media platform? Newer generations of bioprocess professionals are building on the legacies of this industry, and their fresh perspectives are driving innovation. BPI strives to help share that work with as large and relevant an audience as possible — in a range of formats that includes something for everyone. We want to understand how you are most comfortable accessing and sharing technical and business information.
As an editing staff, our preference remains for formal submission and peer review of technical manuscripts. But we’ve been bringing you more interview-based articles, not to bypass technical depth and review, but often because some experts find that approach can present less hassle in corporate legal permissions. By placing the job of manuscript development on the editor, ...
(WWW.STOCK.ADOBE.COM)
To be successful, a company needs two main ingredients: good science and good business/ marketing. Without good science, a product won’t work, and without a good marketing strategy, a product won’t sell. Two important questions should be addressed: When should marketing groups be involved in product development, and how important is that? The answer to the first is
as early as basic research
. Why? After a product is launched, a biomanufacturer doesn’t want to discover that its product applies only to a small market, only a few customers want to buy it, and/or insurance won’t cover it. Marketing due diligence needs to run parallel to product development to derisk a process from a business perspective. It also builds commercial value early in product development (Table 1). Below are six core reasons why.
Table 1:
Product development process (KOL = key opinion leaders)
Defining an Unmet Medical Need
People always ask me, “Should I start with the unmet medical need or the technology?...
When multiple businesses sell the same type of item, why do customers buy from one supplier rather than another? How are vendors able to break through the “white noise” of an industry to stand apart and get noticed? The current bioprocessing and cell therapy vendor markets, about 50,000 vendors are selling to biomanufacturers, universities, and research institutions (www.bioz.com). How do those suppliers get noticed?
Market-leading suppliers have established brands that allow them to cross-sell, up-sell, and engage in deep selling. For everyone else, differentiating through brand strategies is a vastly underused business strategy that, simply put, more companies should engage in — but are not.
In a world filled with increasing digital noise, new vendors and new business ideas fall flat unless they are accompanied by something more than the basic offering. A new market entrant needs something other than an outperforming product or technology to get noticed. Similarly, bioprocessing and cell therapy supply ...
(HTTPS://STOCK.ADOBE.COM)
The growth momentum of China’s biopharmaceutical industry continues, with the China Industry Research Institute projecting that the country’s biological therapeutics market could reach 300 billion Chinese yuan (~US$50 billion) in 2019. According to the second edition of our report on advances in Chinese biopharmaceutical technology, this robust growth is boosted by continuing investment into the sector (
1
). A clear strategic indicator of the country’s intentions in biotherapeutics and biologics has been government investment in bioindustrial hubs, which by next year will have added >$300 billion to the Chinese biological industry nationwide.
As Beijing has continued to build this critical high-technology industry in China over the past decade, it has becoming a major part of both the national economy and the country’s domestic healthcare segment (
1
). Investor confidence has been on the rise since 2014, which has manifested in substantial investments being undertaken in China’...
(HTTPS://STOCK.ADOBE.COM)
The mechanism for proving patent infringement is changing for developers of both branded and follow-on biologics (either biosimilar or interchangeable). Here we examine how drug labeling can establish infringement, thus affecting follow-on manufacturers accused of inducing others to infringe patents on methods of treating medical conditions.
Because precedent is paramount in the US legal system, judges look to small-molecule case history to help them understand alleged infringement by follow-on biologics. The classic approach to induced infringement of generic small-molecule drugs involves an analysis of hypothetical infringement based in part on a prelaunch generic drug label. But a recent case before a well-known US federal judge suggests that an accurate analysis is more nuanced if a drug or biologic already has gone to market. At the time this article was submitted for publication, the case remained pending on appeal. But it has exciting implications for follow-on biologic ma...
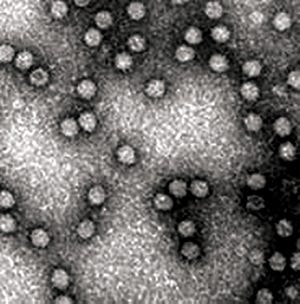
Noninfectious virus-like particles
MOCKV SOLUTIONS (HTTP://MOCKVSOLUTIONS.COM)
Viral safety is a critical focus during biopharmaceutical manufacturing (
1–5
). Although well-characterized mammalian cells such as the Chinese hamster ovary (CHO) line have been used for decades, both endogenous expression of retroviral-like particles and exogenous contamination events from viruses warrant continued vigilance (
6, 7
). International regulatory agencies require biomanufacturers to validate the “viral clearance” efficacy of their downstream manufacturing process steps before resulting products can be awarded clinical trial or commercial approval (
8–10
).
Currently, viral clearance testing is based on small-scale “spiking studies” in which specific model mammalian viruses are introduced artificially (“spiked”) into in-process material and subsequently removed by the specified downstream purification steps (
11, 12
). Spiking studies require specialized biological safety level 2 (BSL-2) laboratories with trained...
The Bio-Process Systems Alliance (BPSA) was formed in 2005 as an industry-led international industry association dedicated to encouraging and accelerating the adoption of single-use manufacturing technologies used in the production of biopharmaceuticals and vaccines. Corporate members include plasticequipment suppliers, service providers, and users in the biopharmaceutical industry who share this mission. A key focus of BPSA’s core activities is to educate its members and others through sharing of information and development of best practice guides that help suppliers, users, and regulators to safeguard the quality of drugs produced with single-use technology (SUT).
(WWW.SHUTTERSTOCK.COM)
Part 2 of this series continues the focus on cell and gene therapy (CGT) manufacturing by providing a regulatory overview. It is based largely on experience gathered from the use of these products in blood processing and biologics manufacturing. Differences between those areas and cell therapies are highlighted throughou...
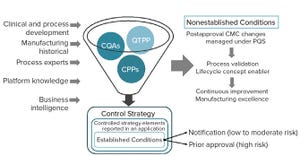
Biopharmaceutical manufacturing processes that were developed before the implementation of quality by design (QbD) typically use control strategies that are not founded on a formal understanding of criticality. Thus, manufacturers of “legacy” products lack the understanding of critical process parameters (CPPs) and critical quality attributes (CQAs). Introducing such elements to a legacy biologic drug product filing aligns fully with expectations described in the ICH Q12 guideline (e.g., increased process understanding and better risk mitigation strategies) (
1
).
Here we discuss how CPPs and CQAs can be defined from the manufacturing history of a legacy commercial drug product. By adding formal risk assessments, manufacturers of such products can improve their filed control strategies significantly and simultaneously provide a science- and risk-based justification for postapproval changes required over the lifecycles of both QbD and non-QbD processes.
ICH Q12 FOR BIOLOGICS
ICH Q12 (
1
) provides biomanuf...
With the increasing adoption of single-use systems (SUS) in critical stages of biopharmaceutical manufacturing, any lack of system integrity can significantly affect drug product quality and patient safety, as well as incur additional costs due to product loss and disrupted production cycle. This article from Sartorius Stedim Biotech, describes how determining the correlation between liquid leakage and microbial ingress can be used to define MALLs (Maximum Allowable Leakage Limits) of SUS for different process steps. The article also details the development of methods and specifications for implementing reliable physical tests to control the integrity of SUS both at a supplier’s site and point of use.
Just fill out the form below to download this article now.
REFERENCES
1
PDA Technical Report 27: Pharmaceutical Package Integrity
. Parenteral Drug Association: Bethesda, MD, 1998.
2
ASTM WK64337: Standard Practice for Integrity Assurance and Testing of Single-Use Systems
. ASTM International: West Consh...
Why is it so hard to develop drugs for children with cancer? And what can be done about it? Those questions are central to a Massachusetts Institute of Technology (MIT) study published in
JAMA
Oncology
exploring new business models for funding drug development to treat pediatric cancers (
1
). Led by Andrew W. Lo (the Charles E. and Susan T. Harris professor at and director of MIT’s Laboratory for Financial Engineering) finds that a collaborative investment structure involving money from private-sector, government, and philanthropic-organization sources holds the greatest promise for new pediatric oncology drug discovery.
“As a new era of drug development dawns,” Lo says, “scientists are finding cures and treatments for different types of cancers every day. But for a variety of reasons, childhood cancers are being left behind. Spurring innovation in this field requires us to be more creative in our approach to financing. And if there is one patient population that really needs our support and advocacy...
As cell and gene therapies approach commercialization, the industry is looking for scalable manufacturing solutions. Equipment providers need to design specifically for the good manufacturing practice (GMP) environment and the unique needs of cell and gene processing. On 3 April 2019, Kevin Lannon of FloDesign Sonics presented an “Ask the Expert” on one company’s solution.
LANNON’S PRESENTATION
In a typical cell therapy manufacturing process, steps that require washing, concentration, or buffer exchange include centrifugation, filtration, and counterflow filtration. Each has inherent concerns such as cell shear, or clogging and fouling. New technologies should address these shortcomings and maintain or improve the quality of cell products being processed.
Acoustophoresis
works by generating a forward-propagating wave that bounces off a reflector, and the two resulting waves together create a standing wave that can be tuned to different strengths. Cells entering the flow path are captured by acoustics int...