- Risk Management
- Regulatory Affairs
- Sponsored Content
Risk Management of Biopharmaceutical Operations: End-to-End and Over LifecycleRisk Management of Biopharmaceutical Operations: End-to-End and Over Lifecycle
Sponsored by 4Tune Engineering
Biopharmaceutical manufacturing processes that were developed before the implementation of quality by design (QbD) typically use control strategies that are not founded on a formal understanding of criticality. Thus, manufacturers of “legacy” products lack the understanding of critical process parameters (CPPs) and critical quality attributes (CQAs). Introducing such elements to a legacy biologic drug product filing aligns fully with expectations described in the ICH Q12 guideline (e.g., increased process understanding and better risk mitigation strategies) (1).
Here we discuss how CPPs and CQAs can be defined from the manufacturing history of a legacy commercial drug product. By adding formal risk assessments, manufacturers of such products can improve their filed control strategies significantly and simultaneously provide a science- and risk-based justification for postapproval changes required over the lifecycles of both QbD and non-QbD processes.
ICH Q12 FOR BIOLOGICS
ICH Q12 (1) provides biomanufacturers incentive to formalize their knowledge assets and improve their lifecycle management strategies. In return, it promises regulatory flexibility with postapproval change management (PACM). The concept of “established conditions” allows companies to categorize process changes into groups and define specific protocols to effect PACM for each group. That requires a knowledge of variation and what is critical to product quality and process performance. It also requires an understanding of how risks can be prevented or mitigated. That requires adequate control strategies as well as knowledge of mechanisms for reviewing risks and opportunities over product lifecycles when current control strategies need improvement.
Biologics have millions of potential candidate CQAs (pCQAs), but only a small fraction of those has clinical relevance. Measuring all pCQAs is impractical, so a tiered-approach — as proposed by the US Food and Drug Administration (FDA) — must be used to rank clinical relevancy and balance residual uncertainty in CQA definition (2, 3). That requires a science- or evidence-based evaluation of available measurable data-driven parts of a process, balanced with a risk-based methodology for uncertain parts.
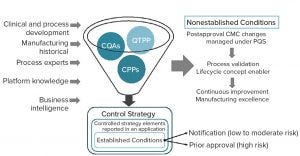
Figure 1: ICH Q12 “established conditions”; CQAs = critical quality attributes, CPPs = critical process parameters, QTPP = quality target product profile
NON-QBD LEGACY FILLINGS AND ICH Q12
We have developed an evidence-driven and risk-based framework for conducting an end-to-end (E2E) criticality assessment of existing commercial processes. This approach uses the regulatory concept of established conditions as set forth in ICH Q12 for data-based parts (Figure 1). Biomanufacturers can map a process E2E and evaluate complex input– output relationships in terms of potential causality obtained from subject-matter experts (SMEs). By crossing those relationships against de facto correlations observed from historical data (Figure 2), manufacturers obtain a critical evaluation of gaps existing in current chemistry, manufacturing, and control (CMC) strategies.
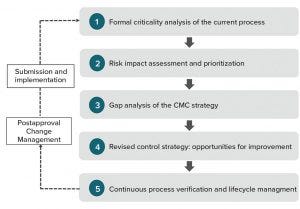
Figure 2: A strategy to build QbD-elements into non-QbD commercial products retrospectively, ensuring continued improvement and change management over lifecycle
SMEs available for legacy processes and products can address such gaps, thus improving filed control strategies. Improvements can take place within proven acceptable ranges (PAR) by fine-tuning of nominal operating ranges (NORs) within filed PAR ranges, therefore not requiring postapproval supplements. Alternatively, given a risk–benefit evaluation, improvements can take place outside PAR ranges. That could justify further investigations (e.g., using scale-down qualified upstream or downstream process models and with specific design of experiments (DoE) and process analytical technology (PAT) tools). Continuous process verification (CPV) and PACM programs then can be built on those systems in a true ICH Q12 sense (4).
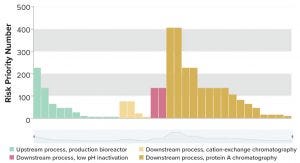
Figure 3: Overview of manufacturing risks end-to-end and over lifecycle for biopharmaceutical operations.
A whole process risk-evaluation E2E can be made at any stage of a lifecycle from process development to industrialization and commercialization (Figure 3). Knowledge about variation, criticality, and causality can help manufacturers revise their control strategies with incremental PACM activities (Table 1).
FULFILLING EXPECTATIONS
How can biomanufacturers align with ICH Q12 guideline expectations? Companies with a clear evidence of quality culture excellence — developing and managing the lifecycles of their products using a science-driven risk-based approach — would benefit the most from the greater regulatory flexibility promised in ICH Q12. For companies that are not quite at that level, the use of quality risk management (QRM) throughout a product lifecycle can ensure consistency within a lifecycle management framework. QRM is strongly connected to knowledge management. So formalizing scienceand evidence-based knowledge, identifying and mitigating risks, and subsequently improving control strategies are the first few steps to take (Figure 2). A lifecycle management plan should include scheduled risk revisions that take into account the operational entirety in an E2E framework and science-based descriptions of historical changes.

Table 1: For risk mitigation and control-strategy improvement, the initial risk priority number (RPN) (red block) was reduced, and the residual risk (yellow block) was considered to be acceptable (S = severity, O = occurence, D = detectability)
After a decade expecting that by applying QbD principles to operations, the biopharmaceutical industry would be compliant by design, biomanufacturers have a sense of significant frustration. The industry now must make a harmonized use of all ICH Q12 regulatory instruments for biomanufacturers to claim some regulatory flexibility. However, for legacy commercial products, QbD elements must be defined and built into non-QbD filings retrospectively by means of the formal risk-management strategy described herein.
REFERENCES
1 ICH Q12: Technical and Regulatory Considerations for Pharmaceutical Product Lifecycle Management (draft). International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use: Geneva, Switzerland, 2017.
2 Guidance for Industry: Clinical Pharmacology Data to Support a Demonstration of Biosimilarity to a Reference Product. US Food and Drug Administration, Silver Spring, MD, 2016.
3 Guidance for Industry: Scientific Considerations in Demonstrating Biosimilarity of a Therapeutic Protein Product to a Reference Product. US Food and Drug Administration: Silver Spring, MD, 2015.
4 Strohmeier M, et al. Knowledge- Based Product and Process Lifecycle Management for Legacy Products. A Lifecycle Approach to Knowledge Excellence in the Biopharmaceutical Industry. Calnan N, Lipa M, Kane P, Menezes JC, Eds. Taylor & Francis, Abingdon, UK, 2017.
José C. Menezes, PhD, is founder and CEO of 4Tune Engineering Ltd. Madalena R. Testas, MSc, Sofia T. Santos, MSc, Silvia C. Ribeiro, MSc, Catarina S. Leitão, MSc, are MS&T specialists at 4Tune Engineering; [email protected]; www.4TuneEngineering.com.
You May Also Like






