November-December 2022
With this issue, we bid a reluctant farewell to our managing editor as she retires. Cheryl and I have known her and appreciated her friendship for 26 years. Her accumulated knowledge of cell and gene therapy development and the ease with which she can interpret the nuances of automation, digitalization, and equations will be sorely missed. To honor her time with us, I asked her to share her perspectives below. Maribel — it has been a thorough joy to work with you!
—S. Anne Montgomery
Maribel Rios,
BPI managing editor
At the end of BPI’s 20th year in publication, I find myself looking back through the different perspectives that many of you have shared in our issues this year and looking forward to new beginnings. Completing this current issue is bittersweet to me because after 26 years of working on pharmaceutical manufacturing publications (13 of those years at BPI), I am retiring. The opportunity to speak with many of you has been my favorite part of the job. The most memorable conversations have been...
Clinical trial inclusivity strengthens analyses, speeds trial completion and Food and Drug Administration (FDA) approvals, and lowers administrative costs of clinical studies. However, a mounting body of evidence shows that eligibility criteria often limit enrollment inclusivity and compromise trial data.
For example, a retrospective review of 302 drug submissions to the FDA examined the impact of insufficient data on drug approvals. Nearly 16% of the reviewed studies had insufficient data to determine safe dosages, and over 13% revealed inconsistent results when alternative endpoints were tested. Over 11% had inconsistent results between study sites, and about 13% failed to demonstrate statistically significant benefits (
1
). As a result, only one product out of every six candidates ultimately gains FDA approval (
2
).
Growing evidence indicates that a lack of diversity among clinical trial participants is a primary cause of inadequate study data and inconsistencies. The good news is that community-base...
The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) released a long-awaited draft revision of its Q9 guidance on quality risk management (QRM) for public consultation in December 2021 (
1
). First published in 2005, ICH Q9 has been instrumental in highlighting the importance of QRM in both the small- and large-molecule pharmaceutical industries. It was the first comprehensive guidance to explain how QRM could be used to identify, assess, and control risks to drug-product quality throughout a pharmaceutical’s life cycle, effectively allowing drug makers to prioritize their resources in specific areas while still maintaining compliance. The document was intended to provide guidance on the application of a level of effort commensurate with the level of risk, and it gave the industry several tools to assist companies on their QRM journeys.
However, implementation of QRM across the pharmaceutical industry over the past 17 years has been difficult, and c...
A multidisciplinary area of research,
synthetic biology
involves the use of genetic engineering to create new biological parts, devices, and systems, with potential applications in industries such as healthcare, agriculture, energy, and environmental science. As early as the 1960s, researchers combined advanced techniques in precision genetic engineering with rational drug development and explored approaches in synthetic biology to support development of innovative drug products. Later research shed new light on how molecular networks regulate cellular function and how gene expression is regulated within cells. Building on that, subsequent advances in molecular biology and DNA sequencing helped to identify and validate strategies that could “program” or engineer bacteria to perform different functions that in turn could deliver therapeutic benefits to patients. In recent years, synthetic biology has become firmly established as a rapidly advancing field of research. It has potential applications in the ...
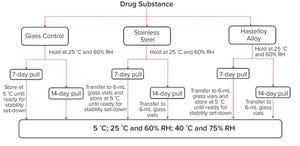
Evaluating compatibility of a drug substance with all surfaces that it might come into contact with during drug product manufacturing is essential to ensure product quality. Proteins can adsorb to contact surfaces, form aggregates, and desorb into a drug-substance solution. Proteins also can degrade in presence of leachables generated from contact surfaces during manufacturing.
Containers and vessels used during manufacturing are single-use disposable components or metal tanks, primarily either 316L stainless steel (SS) or C-276 Hastelloy nickel-based alloy (HLY). Researchers have shown protein degradation in presence of metal contact surfaces. In two separate studies, Bee et al. (
1
) and Kalonia et al. (
2
) reported adsorption of monoclonal antibodies (MAbs) on SS surfaces and subsequent aggregation, attributing the phenomenon to structural alteration or unfolding caused by the adsorption step. Different formulation factors (e.g., buffer species, pH, volume per unit contact surface area, protein concen...
Proteins inside and on the surfaces of Gram-negative bacteria easily can trigger immune responses to therapeutic proteins in biologic products.
(
HTTPS://ALAMY.COM
)
Rigorous physicochemical and bioanalytical methods must be performed on biological products to ensure that they contain minimal levels of host cell proteins (HCPs) and other process-related impurities. In the first and second parts of our article, we surveyed literature about HCPs of concern, the mechanisms behind their immunogenicity, and ultimately, their consequences for patient safety. Herein, we highlight published case studies to explore difficulties with detecting, identifying, and quantifying such impurities. These examples demonstrate that much remains to be learned about how HCPs relate to immune responses and how to predict immunogenicity potential. New information and improvements to in vitro assays could enhance prevention of now-undetectable safety issues.
The First Associations of Therapeutic Proteins and HCPs
HCPs remain an i...
Since its inception four decades ago, cell-free synthesis (CFS) has been used to produce biomolecules such as RNA, DNA, peptides, and proteins (
1
). However, most of these applications have been in early stage research and small-scale proof-of-concept studies, with rare examples of large-scale production. The slow industrial uptake of CFS has been attributed to low productivity, which suggests an uneconomical path to large-scale manufacture.
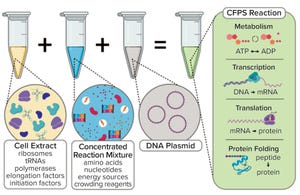
Figure 1a:
Generic illustration of the key components in a cell-free protein synthesis (CFPS) reaction (
4
).
Typically, a CFS platform includes a genetic template (encoding the product of interest), chemical additives (nucleotides and an energy source), and a lysate (extract from ruptured cells) as prerequisites for the upstream reaction (Figure 1a). Recently, interest has grown in considering cell-free technology as a potentially attractive production platform for stratified medicine and rare disease treatments (
2
), on-demand medicine (
3–5
), highly potent mole...
Cell therapies are promising new drug products that treat or cure diseases that, until the past decade, had no other treatment options. Several autologous cell therapies have been approved, and their efficacy has been proven, especially in immunooncology. However, autologous therapies can present some difficulties for both developers and patients (e.g., short timelines, point-of-care drug administration). Allogeneic cell therapies are not associated with those challenges. For example, patient access to an autologous treatment can take months, time that patients with fast-progressing diseases might not have.
Having an allogeneic cell therapy that is ready to use immediately is advantageous. Starting materials for autologous cell therapies often are suboptimal because of immune-cell exhaustion and the health of the patients from which those materials are sourced. Cells manufactured for allogeneic therapies can be sourced from healthy donors and be fully characterized. Thus, the risk of patient-to-patient va...
Photo 1:
PendoTECH TFF process control system benchtop configuration.
PendoTECH tangential-flow filtration (TFF) process control systems have been widely adopted in the filtration community. The system’s features enable its implementation in multiple unit operations and biopharmaceutical processing applications, including ultrafiltration–diafiltration (UF–DF) of proteins, viruses, and compounds such as oligonucleotides and antibody–drug conjugates (ADCs). The control system can be used to develop process parameters for UF–DF processes that have different membrane formats (e.g., flat-sheet cassette and hollow fiber).
TFF laboratory setups often are manual or semiautomated processes that use pumps, stainless-steel pressure gauges or transmitters, scales, and manual valves. Data logging requires continuous monitoring, and exporting data requires laborious efforts to transfer to data analysis software. Integrating multiple system components from different suppliers and using manually operated clamps and valv...
During a September 2022 webinar, Kyle Deluca (a senior scientist in engineering at Merck) highlighted bottlenecks in his group’s workflow for characterizing production of a pneumonia vaccine. In that process, purified components undergo chemical modification in a synthesis reactor, followed by buffer exchange, size-based separation, additional chemical modification, and final purification. Because the modification steps were time and labor intensive, Merck automated them. With support from Mettler Toledo, Deluca and Felix Milman (a specialist in engineering at Merck) described how their group implemented the EasyMax 102 thermostat system and associated software tools, improving process consistency and robustness while reducing time and labor requirements.
The Presentation
Deluca explained that both chemical-modification steps involve precision dilution and dosing of buffers and reagents, incubation within a narrow temperature range over a long period, and subsequent cooling of reaction products. Initial w...
Biopharmaceuticals delivered by viral vectors (VVs) face distinctive obstacles during early upstream development. In October 2022, Andres Castillo (a portfolio manager at Sartorius) noted that drug makers set short development timelines to hasten therapies into the clinical evaluation. Doing so limits time for analyzing complex biointeractions, and studies for culture-media and VV screening are time- and resource-intensive. Castillo and Shanya Jiang (also a portfolio manager at Sartorius) explored how integrated technologies facilitate robust cell-line and VV screening.
The Presentation
Reflecting on the complexity of upstream development, Castillo said, “It is imperative to leverage systems that make initial data collection easier and more robust to facilitate scale-up.” Sartorius has established an ensemble of culture and bioanalytical solutions to help users perform comprehensive testing, evaluate data, and apply resulting insights to characterize critical quality attributes and process parameters (CQA...
Biopharmaceutical manufacturers usually apply resin-based affinity- chromatography media for monoclonal-antibody (MAb) capture. Such materials are costly, and their biophysical limitations can create operational difficulties. In an October 2022 webinar, Volkmar Thom (director of membrane chromatography R&D at Sartorius) spoke about his company’s development of a “convecdiff” affinity membrane. He described how the technology can help users to intensify capture processes, reducing downstream manufacturing costs.
The Presentation
Protein A resins contain porous beads of 50–100 μm in diameter. MAbs must diffuse into the pores for a resin to maximize its dynamic binding capacity (DBC). But such spaces have diffusional pathlengths (25–50 μm), and MAbs have relatively large hydrodynamic diameters (~10 nm). Thus, MAb solutions must undergo long residence times for a resin to achieve reasonable binding capacity, resulting in low process productivity (e.g., 10–20 g MAb/L resin/h). Lengthy processes prevent operato...
The personalization of autologous advanced therapy medicinal products (ATMPs) makes patients an intrinsic part of the supply chain. It begins with collection and registration of starting materials, which necessitates tracking and recording chain-of-identity (CoI) and chain-of-custody (CoC) information for every dose. This situation could expand the challenges of patient data management throughout the whole supply chain, increasing data privacy risks to both patients and suppliers. ATMP manufacturers must therefore protect data privacy not only as clinical trials start, but also as therapies extend into additional territories where data and documentation requirements often differ.
The CoI challenge that ATMP developers have become familiar with grows significantly more complex when they scale therapies commercially. Working with increasing numbers of suppliers from different geographic areas complicates both supply chain orchestration processes and communication and enforcement of regulatory criteria.
Glob...