Opportunities in the Field of Host Cell Proteins Part 3: Case Studies in Impurity Detection and IdentificationOpportunities in the Field of Host Cell Proteins Part 3: Case Studies in Impurity Detection and Identification
Proteins inside and on the surfaces of Gram-negative bacteria easily can trigger immune responses to therapeutic proteins in biologic products. (HTTPS://ALAMY.COM)
Rigorous physicochemical and bioanalytical methods must be performed on biological products to ensure that they contain minimal levels of host cell proteins (HCPs) and other process-related impurities. In the first and second parts of our article, we surveyed literature about HCPs of concern, the mechanisms behind their immunogenicity, and ultimately, their consequences for patient safety. Herein, we highlight published case studies to explore difficulties with detecting, identifying, and quantifying such impurities. These examples demonstrate that much remains to be learned about how HCPs relate to immune responses and how to predict immunogenicity potential. New information and improvements to in vitro assays could enhance prevention of now-undetectable safety issues.
The First Associations of Therapeutic Proteins and HCPs
HCPs remain an important issue in biologics development. Despite almost 40 years of commercial availability, biologics still lack an official range for HCP limits. Careful reading helps to understand the origin of such concerns.
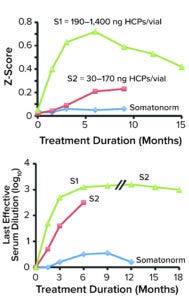
Figure 1: Presence of Escherichia coli polypeptide (ECP) in a recombinant human growth hormone (rhGH) product correlates with induction of anti-rhGH antibodies in treated patients, with evidence of adjuvant effect. The top panel plots out numbers of anti-ECP antibodies, represented by Z-scores, in patients treated with preparations of somatren and ECP (S1, S2, and Somatonorm). Each Z-score is the quotient of anti-ECP antibodies in treated patients divided by anti-ECP antibodies in standard serum. The bottom panel shows log10 values of the last effective serum dilution of each preparation. (Figure adapted from 19.)
Initial analyses of HCPs in recombinant-protein drug products were performed by Bierich’s team in 1986 (19). They studied three preparations of recombinant human growth hormone (rhGH) administered to hypopituitary children. Samples contained different amounts of an HCP called Escherichia coli polypeptide (ECP): 190–1400 ng of ECP/vial (S1), 30–170 ng/vial (S2), and 2–10 ng/vial of Somatonorm (somatren, Kabi Vitrum) (S3). The team determined that anti-ECP antibodies were induced mainly in children who received the S1 and S2 preparations. The highest number of anti-ECP antibodies was recorded for S1 at six months after administration, with a Z-score of >0.7. Thus, the results demonstrated that ECP concentration correlated with generation of anti-ECP and anti-rhGH antibodies (Figure 1).
Formulation S1, which contained the highest ECP concentration and generated the greatest numbers of anti-rhGH antibodies after administration, induced the highest growth rates among treated children. It also recorded a slightly lower standard deviation (SD) than did S2 (but still showed a higher SD than did the somatrem product). As the team reported in the study, “The best results are found with preparation S1.”
However, during short-term phase 1 clinical trials, scientists noticed severe allergic skin reactions at the site of injection (intramuscular) in two of the 28 patients who received S1. No allergic responses were reported during experiments for S2 or S3.
Bacterial proteins such as peptidoglycan, flagellin, and fimbria and pili proteins are known to induce strong immunogenic responses when they bind to toll-like receptors (TLRs). Palm and Medzhitov have shown that the nonimmunogenic protein human serum albumin (HSA) mixed with lipopolysaccharide (LPS) can induce immunogenic antibody responses via TLR4 signaling (121). Notably, haptenated proteins can induce independent T-cell responses. In the same set of experiments, Palm and Medzhitov demonstrated that haptenated HSA can induce robust CD4+ T-cell and antibody IgG1 and IgG2c responses to dinitrophenylated HSA (DNP-HSA) but not to standard HSA.
Regarding Bierich, it still is unclear whether anti-rhGH antibodies were induced by protein aggregates, N-terminal methionine, or ECP via TLRs. Also, those experiments did not include the same number of patients: 12 children (rather than 28) received preparation S2, and nine received S3. There is no way to discount bias in allergic induction in the recruited patients because such reactions are expected to occur among large population samples (130).
Contemporary studies on protein immunogenicity point out that aggregation is the main factor by which immune response and antidrug antibodies (ADAs) can be triggered against rhGH and other biotherapeutics (130–135). The experimental basis for that claim rests in observations by Mitchinson (136). A bovine serum albumin (BSA) solution was centrifuged overnight at high speed. The next day, supernatant was collected and administered intravenously to rabbits, with the result of immune tolerance. Later, the pellet was resuspended and injected into rabbits, inducing a strong anti-BSA response.
Whether HCPs and/or aggregates were purified from samples in Bierich’s study cannot be verified. It is worth noting, however, that those E. coli–derived rhGH preparations were N-terminal formylmethionine-GH molecules (137). Such proteins are known to be immunogenic (138) and to pose threats to protein stability (139). Thus, N-terminal amino acids usually are clipped off of therapeutic proteins during manufacture using various methioninepeptidases, aminopeptidases, or leucine aminopeptidases (140, 141).
Another interesting consideration for Bierich’s study is that the somatrem product (S3) did not induce neutralizing ADAs. The study author argues that ECP induced the immunogenic reactions, probably through adjuvant activity similar to that of live vaccines. Indeed, Freund’s adjuvant (an emulsion of lyophilized Mycobacterium tuberculosis) (60), monophosphoryl lipid A (MPL-A), LPS, detoxified LPS, and lipopeptides all can induce strong immunogenic cross-reactions with nonimmunogenic proteins (121). Perhaps, then, we have a new immunogenicity induction model. Moreover, HSA and LPS are emulsified with incomplete Freund’s adjuvant (lacking M. tuberculosis), which can aid in adjuvant responses. Other studies on vaccines point out that efficient induction of a cross-reaction with an immunogenic protein requires that the protein be present in nanoparticles mixed with poly(lactic-co-glycolic acid) (PLGA) (142), with or without MPL-A (an inductor of TLR4), and throughout antigens that can activate multiple TLRs (e.g., TLR4 and TLR7). Otherwise, antibody titers are insignificant (143).
Huang et al. describe a biological assay in which human embryonic kidney (HEK) cells (which have active TLRs) were activated by an unspecified sample, inducing expression of interleukin (IL)-8 and IL-6 at levels similar that of a sample spiked with flagellin (76). That result indicates that TLRs can be activated by HCPs.
This is just a speculation, but it is possible that, during Bierich’s study, the interaction of folded/unfolded/aggregated rhGH with ECP formed complexes that went on to stimulate production of binding antibodies (54). Indeed, misfolded proteins and aggregates always will induce immunogenic responses. Although the pathway is not well understood, marginal zone B (MZB) cells are related to such T-cell–independent responses (144). Ultimately, the nature of an ECP can be critical to induction of anti-rhGH antibodies. Further studies of ECP identity, neutralizing antibodies (NAbs), and rhGH complexes and/or aggregates will help to shed light on the relation of HCPs to immune responses.
Somatrem products largely have been replaced by rhGHs that are nearly identical to native hormones. Based on the evidence presented herein, it seems that the immunogenic nature of HCPs played a critical role in the Bierich case. But aside from atopic allergic reactions, patients reported no other adverse reactions to their respective treatments.
HCPs in a Filgrastim Product
Wadhwa and collaborators investigated the composition of two filgrastim products (18). A recombinant version of granulocyte-macrophage colony-stimulating factor (GM-CSF), filgrastim is used to enhance proliferation and differentiation of hematopoietic progenitor cells and to modulate the effector functions of monocytes, neutrophils, and mononuclear cells (18, 145). The protein is overexpressed in E. coli cells. Wadhwa’s team established that one product induced ~95% of NAbs, whereas the other induced ~74%. In vitro pretreatment of the second GM-CSF product with antisera from patients who had received the treatment produced antibodies against proteins unrelated to GM-CSF — HCPs. Therefore, NAbs seem to be related to HCPs in this case.
The impurities were determined to have molecular weights of about 20 kDa and 30 kDa. The researchers also found that the two E. coli–derived proteins had no effect on GM-CSF performance; rather, only antibodies that neutralized GM-CSF compromised a product’s clinical response. Although not mentioned in the study, products can have N-terminal methionine, which could be the main reason for NAb induction (139). Moreover, proteins expressed in E. coli can be insoluble or inactive because of the absence of glycosylation (146, 147). Such factors can lead to aggregation of GM-CSF, a labile protein. Anaphylactoid adverse effects were not observed in Wadhwa et al. (18). That article, often cited in literature, contradicts the adjuvant theory of HCP immunogenicity. However, the role of HCPs in adjuvant effects for induction of ADAs could be an interesting area of inquiry.
HCPs in a Biosimilar Somatropin
Early in the development of Sandoz’s Omnitrope product, a biosimilar rhGH, clinical data showed that 57% of patients who received it developed nonneutralizing antibodies, and all patients developed anti-HCP antibodies (75). Subsequent efforts to reduce HCP levels proved to be successful in lowering ADAs to 2%, a level comparable to that of the innovator product, Pfizer’s Genotropin (somatropin). Adjuvant effect was attributed to the presence of a ribose phosphate isomerase identified by liquid chromatography with tandem mass spectrometry (LC-MS/MS) at an estimated level of 1,400 ng/mg of protein. Immunoblots showed only one band, implying the presence of a single impurity (148).
However, at the time, no official reference could be followed, nor were there official guides to know how analytical and immunological methods were to be executed. Again, the adjuvant effect of HCPs against N-terminal-methionyl-rhGH is not well understood. Extended purification protocols can eliminate rhGH aggregates (130); oxidation, cyclization, or elimination reactions (149); and unfolded structures of rhGH fragments — which can induce ADAs just like HCP–rhGH complexes/aggregates can, although that was not demonstrated in the study. (The same can be said for LPS and CpG DNA, which are not proteins).
The writers of the Omnitrope study mention that complete characterization was executed, and they concluded that the key difference between the immunogenic and optimized (nonimmunogenic) formulations was the amount of HCPs present (148). However, there is no way to verify that information or reproduce those experiments. In fact, no other examples can be found of NAb cross-reactivity between HCPs and therapeutic proteins.
Here, it is worth mentioning the Jones et al. results (84). When studying an N-terminal methionine hGH, they found that patient immunogenic responses diminished, with no safety consequences related to antibody formation, after additional purification steps were introduced into the product’s manufacturing process. The study supported that “immunogenicity was not due to N-terminal methionine nor E. coli protein impurities but small amounts of rhGH with subtle structural alterations” (84). That conclusion suggests that therapeutic protein aggregates are relevant to immunogenic issues. Aggregates of therapeutic proteins can induce independent T-cell responses (149). And as Palm and Medzhitov showed, aggregated complexes of host-cell and therapeutic proteins can be highly immunogenic (121). But after 40 years of biologic availability on the market, to the best of our knowledge, no investigation has been performed to demonstrate the adjuvant HCP hypothesis directly.
The previously cited articles have key pieces of information in common, however. Reports on rhGH derived from E. coli expression systems showed that HCPs resulted in ADAs, whereas studies of products that were expressed in Chinese hamster ovary (CHO) cells showed lower immunogenic induction, but with biological activity. These observations indicate that E. coli HCPs induce immune responses more “easily” than those from eukaryotic hosts.
A Study of T-Cell Proliferation
Information relating to potential adjuvant effects from interactions between host-cell and therapeutic proteins has remained conjectural and elusive. Thus, a group of scientists from Amgen in Thousand Oaks, CA, designed and executed experiments focused on the interrelation of HCPs and therapeutic proteins. Antigens (Ags) induce T-cell proliferation in a process called clonal expansion through autocrine and paracrine signaling. T-cell proliferation is associated directly with adaptative cellular responses and inflammatory processes. Jawa et al. induced T-cell proliferation using an in vitro comparative immunogenicity assessment (IVCIA) assay using three different MAbs mixed with different HCPs quantities ranging from 4,258 ppm to 396 ppm (150). Those impurities represented 139 proteins from CHO cells. The MAbs were purified using hydrophobic-interaction chromatography, and HCPs levels were reduced to between 44 ppm and undetectable levels.
IVCIA testing demonstrated no differential T-cell proliferation between MAb samples with high and low HCP concentrations (Figure 2). Even levels of ~4,300 ng HCPs/mg of MAb induced no more pronounced immune responses than were observed with the therapeutic protein itself.
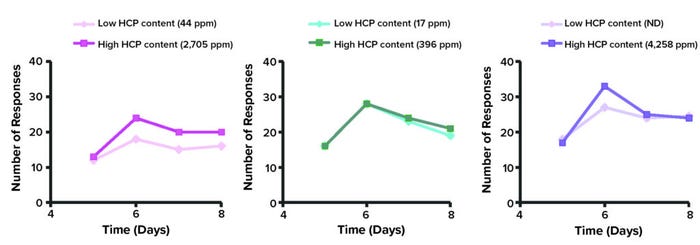
Figure 2: In vitro comparative immunogenicity assessment (IVCIA) in healthy humans after five to eight days of treatment with different monoclonal antibodies (MAbs); the traces below represent signals from tritiated thymidine captured by T cells after incubation with samples of “MAb 2” mixed with varied levels of host cell proteins (HCPs). (Figure adapted from 150.)
That experiment is exemplary; however, it did not undertake identification of specific HCPs — and, as we know, the presence of a particular impurity can be critical to induction of immunogenic responses. Moreover, a low immunogenic response should be expected from this type of method because human subjects are not exposed to CHO cells under typical conditions and because CHO cells are not infective organisms with pathogen-associated molecular pattern molecules (PAMPs) present on their surfaces (also, cross-reactions with alarmins are not expected).
An Example of a Safe HCP
It is generally accepted that HCP levels in a drug substance must be diminished to 1–100 ppm (24, 25). However, exceptions are possible. Epoetin Hospira is a biosimilar of the innovator Epogen product, a recombinant human erythropoietin (rhEPO). The US Food and Drug Administration (FDA) approved the biosimilar for commercialization in May 2018. HCP content makes up 0.2–0.3% the drug product, which is ~2,000–3,000 ppm
(ng HCPs/mg rhEPO) as reported by reversed-phase high-performance liquid chromatography (RP-HPLC) (151, 152).
The FDA asked Hospira for information about the identity of HCPs in the drug product and about the validation of analytical-method accuracy and reproducibility (153). The manufacturer identified one impurity as “olfactory receptor protein” using RP-HPLC-MS and peptide mapping based on trypsin digestion.
The agency required application of appropriate warnings on the Epoetin product label, including notes about possibilities for “serious allergic reactions” and “severe cutaneous reactions.” The FDA also mandated development of a follow-on record (pharmacovigilance) about adverse events induced by the drug product (154). However, since then, no reports have been submitted about Retacrit anaphylactoid adverse responses, suggesting that the HCP might not be dangerous to patients.
Phospholipase B-like Protein 2 (PLBL2)
This protein is known to bind and copurify with several humanized antibodies. Genentech (now part of Roche) scientists who wrote about HCPs in a lebrikizumab product mention that although administration of the product during early clinical testing generated antibody responses against CHO protein PLBL2, no deleterious immune-related anaphylactoid or allergic effects were elicited in patients (155). Nevertheless, after clinical phase 2, Genentech improved the product’s purification processes to diminish PLBL2 levels further. During phase 2b studies, trial participants were exposed to ~9.1–82 µg/dose (37.5–250 mg of lebrikizumab/month). Such levels showed no impact on lebrikizumab immunogenicity, and no subsequent effects came from the identified anti-PLBL2 antibodies. Of particular interest here is that, according to the authors, the PLBL2 impurity cannot be detected by an enzyme-linked immunosorbent assay (ELISA).
Toward Best Practices for HCP Detection and Identification
This third part of our article has explored cases of HCP detection to highlight what we know — and what we must learn more about — regarding the mechanisms behind immunogenicity of HCPs. We will conclude our article in the January–February 2023 issue of BPI with discussion of best practices and a look ahead to the future of HCP analytics.
Acknowledgments
This work was supported by Mexico’s Consejo Nacional de Ciencia y Tecnología (CONACyT), Grant FINNOVA-CONACyT 174104. Authors VPMM and NOP are Sistema Nacional de Investigadores (SNI)-L1 CONACyT Fellows.
Disclosures
Probiomed S.A. de C.V. develops, manufactures, and markets biosimilar products. All three authors are involved in the development of biosimilar products for Probiomed.
References
See part 1 for references 1–34 and part 2 for references 35–129.
18 Wadhwa M, et al. Immunogenicity of Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF) Products in Patients Undergoing Combination Therapy with GM-CSF. Clin. Cancer Res. 5(6) 1999: 1353–1361; https://pubmed.ncbi.nlm.nih.gov/10389919.
19 Bierich JR. Treatment of Pituitary Dwarfism with Biosynthetic Growth Hormone. Acta Paediatr. Scand. 75(s325) 1986: 13s–18s; https://doi.org/10.1111/j.1651-2227.1986.tb10357.x.
24 Chon JH, Zarbis-Papastoitsis G. Advances in the Production and Downstream Processing of Antibodies. New Biotechnol. 28(5) 2011: 458–463; https://doi.org/10.1016/j.nbt.2011.03.015.
25 Champion K, et al. Defining Your Product Profile and Maintaining Control Over It, Part 2. BioProcess Int. 3(8) 2005: 52–57; https://bioprocessintl.com/2005/september-2005/defining-product-profile-maintaining-control-part-2.
54 Kumar H, Kawai T, Akira S. Pathogen Recognition by the Innate Immune System. Int. Rev. Immunol. 30(1) 2011: 16–34; https://doi.org/10.3109/08830185.2010.529976.
60 Hatai H, et al. Toll-Like Receptor 11 (TLR11) Interacts with Flagellin and Profilin Through Disparate Mechanisms. PLOS one 11(2) 2016: e0148987; https://doi.org/10.1371/journal.pone.0148987.
75 Gelzleichter TR. Early Characterization of Biosimilar Therapeutics. Nonclinical Development of Novel Biologics, Biosimilars, Vaccines and Specialty Biologics. Plitnick L, Herzyk D, Eds. Academic Press: Cambridge, MA, 2013: 185–210.
76 Huang LY, et al. Use of Toll-Like Receptor Assays to Detect and Identify Microbial Contaminants in Biological Products. J. Clin. Microbiol. 47(11) 2009: 3427–3434; https://doi.org/10.1128/JCM.00373-09.
84 Jones AJ. The Use of an Animal Immunogenicity Model in the Development of Protropin Somatrem (Methionyl Human Growth Hormone). Dev. Biol. (Basel) 109, 2002: 107–118.
121 Palm NW, Medzhitov R. Immunostimulatory Activity of Haptenated Proteins. Proc. Nat. Acad. Sci. USA 106(12) 2009: 4782–4787; https://doi.org/10.1073/pnas.0809403105.
130 Fradkin AH, Carpenter JF, Randolph TW. Immunogenicity of Aggregates of Recombinant Human Growth Hormone in Mouse Models. J. Pharm. Sci. 98(9) 2009: 3247–3264; https://doi.org/10.1002/jps.21834.
131 Lundahl M, et al. Aggregation of Protein Therapeutics Enhances Their Immunogenicity: Causes and Mitigation Strategies. RSC Chem. Biol. 2(4) 2021: 1004–1020; https://doi.org/10.1039/d1cb00067e.
132 Moore WV, Leppert P. Role of Aggregated Human Growth Hormone (hGH) in Development of Antibodies to hGH. J. Clin. Endocrinol. Metab. 51(4) 1980: 691–697; https://doi.org/10.1210/jcem-51-4-691.
133 Schellekens H. How To Predict and Prevent the Immunogenicity of Therapeutic Proteins. Biotechnol. Ann. Rev. 14, 2008: 191–202; https://doi.org/10.1016/S1387-2656(08)00007-0.
134 Rosenberg AS. Effects of Protein Aggregates: An Immunologic Perspective. AAPS J. 8(3) 2006: E501–507; https://doi.org/10.1208/aapsj080359.
135 Ratanji KD, et al. Immunogenicity of Therapeutic Proteins: Influence of Aggregation. J. Immunotoxicol. 11(2) 2014: 99–109; https://doi.org/10.3109/1547691X.2013.821564.
136 Mitchinson NA. Induction of Immunological Paralysis in Two Zones of Dosage. Proc. Royal Soc. B Biol. Sci. (London) 15 December 1965: 275–292; https://doi.org/10.1098/rspb.1964.0093.
137 Furman BL. Somatrem. xPharm: The Comprehensive Pharmacology Reference. Enna SJ, Bylund DB, Eds. Elsevier: Amsterdam, The Netherlands, 2011.
138 Bufe B, et al. Recognition of Bacterial Signal Peptides By Mammalian Formyl Peptide Receptors: A New Mechanism for Sensing Pathogens. J. Biol. Chem. 290(12) 2015: 7369–7387; https://doi.org/10.1074/jbc.M114.626747.
139 Varshavsky A. The N-End Rule: Functions, Mysteries, Uses. Proc. Nat. Acad. Sci. (USA) 93(22) 1996: 12142–12149; https://doi.org/10.1073/pnas.93.22.12142.
140 Liao YD, et al. Removal of N-terminal Methionine from Recombinant Proteins By Engineered E. coli Methionine Aminopeptidase. Protein sci. 13(7) 2004: 1802–1810; https://doi.org/10.1110/ps.04679104.
141 Slavchenko I. et al. Overexpression and Purification of Methionine Aminopeptidase from Escherichia coli. Biopolymers and Cell 19(3) 2003: 274–280; http://dx.doi.org/10.7124/bc.00065C.
142 Athanasiou E, et al. A Poly(Lactic-co-Glycolic) Acid Nanovaccine Based on Chimeric Peptides from Different Leishmania infantum Proteins Induces Dendritic Cells Maturation and Promotes Peptide-Specific IFNγ-Producing CD8+ T Cells Essential for the Protection Against Experimental Visceral Leishmaniasis. Front. Immunol. 8, 2017: 684; https://doi.org/10.3389/fimmu.2017.00684.
143 Kasturi SP, et al. Programming the Magnitude and Persistence of Antibody Responses with Innate Immunity. Nature 470(7335) 2011: 543–547; https://doi.org/10.1038/nature09737.
144 Gatto D, et al. Rapid Response of Marginal Zone B Cells to Viral Particles. J. Immunol. 173(7) 2004: 4308–4316; https://doi.org/10.4049/jimmunol.173.7.4308.
145 Castellani S, et al. G-CSF and GM-CSF Modify Neutrophil Functions at Concentrations Found in Cystic Fibrosis. Sci. Rep. 9, 2019: 12937; https://doi.org/10.1038/s41598-019-49419-z.
146 Conradt HS, et al. Structure of the Carbohydrate Moiety of Human Interferon-Beta Secreted By a Recombinant Chinese Hamster Ovary Cell Line. J. Biol. Chem. 262(30) 1987: 14600–14605; https://doi.org/10.1016/s0021-9258(18)47838-6.
147 Wu JJ, Choi LE, Guidotti G. N-Linked Oligosaccharides Affect the Enzymatic Activity of CD39: Diverse Interactions Between Seven N-Linked Glycosylation Sites. Molec. Biol. Cell 16(4) 2005: 1661–1672; https://doi:10.1091/mbc.e04-10-0886.
148 Vanderlaan M, et al. Experience with Host Cell Protein Impurities in Biopharmaceuticals. Biotechnol. Prog. 34(4) 2018: 828–837; https://doi.org/10.1002/btpr.2640.
149 Grassi L, Cabrele C. Susceptibility of Protein Therapeutics to Spontaneous Chemical Modifications By Oxidation, Cyclization, and Elimination Reactions. Amino Acids 51(10–12) 2019: 1409–1431; https://doi.org/10.1007/s00726-019-02787-2.
150 Martin F, Oliver A, Kearney J. Marginal Zone and B1 B cells Unite in the Early Response Against T-Independent Blood-Borne Particulate Antigens. Immunity 14(5) 2001: 617–629; https://doi.org/10.1016/s1074-7613(01)00129-7.
151 Jawa V, et al. Evaluating Immunogenicity Risk Due to Host Cell Protein Impurities in Antibody-Based Biotherapeutics. AAPS J. 18(6) 2016: 1439–1452; https://doi.org/10.1208/s12248-016-9948-4.
152 Lugo MG, Namuswe F. BLA STN 125545: Addendum for “Epoetin Hospira” Drug Substance Shelf-Life. US Food and Drug Administration: Silver Spring, MD, 2018; https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/125545Orig1s000ChemR.pdf.
153 De Claro RA. BLA Approval: Retacrit (epoetin alfa-epbx). US Food and Drug Administration: Silver Spring, MD, 2018; https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2018/125545Orig1s000ltr.pdf.
154 FDA Approves First Epoetin Alfa Biosimilar for the Treatment of Anemia (press release). US Food and Drug Administration: Silver Spring, MD, 15 May 2018; https://www.fda.gov/news-events/press-announcements/fda-approves-first-epoetin-alfa-biosimilar-treatment-anemia.
155 Fischer SK, et al. Specific Immune Response to Phospholipase B-Like 2 Protein, a Host Cell Impurity in Lebrikizumab Clinical Material. AAPS J. 19(1) 2017: 254–263; https://doi.org/10.1208/s12248-016-9998-7.
Víctor Pérez Medina Martínez and Carlos Eduardo Espinosa-de la Garza work in the research and development unit, and corresponding author Néstor O. Pérez works in operations management at Probiomed S.A. de C.V., Cruce de Carreteras Acatzingo-Zumpahuacán s/n, Tenancingo, Estado de México, México. C.P. 52400; 52-55-1166-2305; [email protected]; https://www.probiomed.com.mx.
You May Also Like





