August 2016 Supplement
O
ur second annual BPI Theaters supplement once again brings you summaries from
WWW.LIFETOUCH.COM
presentations at our BioProcess Theaters during Interphex and the BIO Convention.As a summer companion piece to our annual Yearbook’s company profiles and application notes, this serves as a midyear overview of the biopharmaceutical landscape.
These summaries are the highlights.To hear the full presentations (and see their PowerPoint slides) please go to
www.bioprocessintl.com/Interphex2016
www.bioprocessintl.com/BIO2016
This special summer issue comes to you through the efforts of many people.Our publisher, Brian Caine, and his team created two excellent programs, each focused on the interests of a majority of the particular event’s attendees.Sincere thanks also go to the speakers — who graciously contend each year with exhibit-hall noise, uncertain attendance, and occasional loudspeaker interruptions.Their efforts are rewarded by lively conversations after the presentations.Inclusion in this issue and on ou...
A BPI Theater Roundtable at Interphex 2016
SANOFI(WWW.SANOFI.COM)
On Tuesday, 26 April 2016, Eric S. Langer (managing partner of BioPlan Associates) chaired a morning roundtable titled “Deciding on Single-Use vs. Stainless Steel Strategy: What the CMOs Know That Biopharma Needs” as a follow-up to a similar discussion held last year. Langer brought together a panel of four contract manufacturing organization (CMO) industry experts:
Langer began this session by asking, “What do CMOs really know, and how can they share that with end users?” He introduced the panel as a “brain trust” of bioprocessing and single-use technology knowledge. CMOs are paid to be more efficient and test new technologies. Bioprocessors have been trying to decide whether to stay with stainless steel equipment or switch to single-use technologies. But they have come to realize that it isn’t an either–or decision. Integration of both approaches depends on product volume and the amount of flexibility needed. Stainless steel is still the ...
A BPI Theater Roundtable at Interphex 2016
On Tuesday, 26 April 2016, James D. Vogel (founder and director of The BioProcess Institute at the University of Rhode Island) chaired a midday roundtable titled, “Single-Use Harmonization Town Hall: Coordination of SUS Standards and Best Practice Efforts.” Two industry experts joined him in a panel discussion:
All three are members of different groups interested in developing standards for single-use technologies. Nicknamed “the alphabet-soup” groups, they include the BioProcess Systems Alliance (BPSA), BioPhorum Operations Group (BPOG), Parenteral Drug Association (PDA), International Society for Pharmaceutical Engineering (ISPE), Single-Use Technical Assessment Program (SUTAP), American Society of Mechanical Engineers (ASME), BioPharma Engineering (BPE), United States Pharmacopeia (USP), and the American Society for Testing and Materials (ASTM).
Vogel said that standards organizations are looking for answers to the following questions: What are you interested ...
Distek’s BIOne system (
WWW.DISTEKINC.COM
)
Mark Arjona
(product line manager, Distek) 1:00–1:20 pm
The bioprocessing industry has embraced single-use technologies recognizing their benefits of safety, cost, and throughput. Based in New Jersey, Distek is a leading manufacturer of single-use equipment.
The BIOne is a single-use bioreactor system consisting of a patent-pending, disposable headplate and liner that fit together into a bench-scale glass vessel. The liner molds to the contours of culture vessels (3 L or 7 L), keeping their geometry, aspect ratio, and volume all the same. The single-use headplate is prefitted with tubing, has the same ports, and can interface with a controller in the same way a stainless steel headplate does. But it does not need to be autoclaved. That allows users to quickly convert their traditional systems to single use while using an existing vessel, controller, probes, and agitator. By eliminating the cleaning and assembly steps of a traditional (reusable) set-up, users ca...
Jim Furey
(general manager, PendoTECH) BPI Theater @ INTERPHEX, April 26, 2016 11:30 am–12:00 pm
Located in Princeton, NJ, PendoTECH was founded in 2005. The company develops equipment for single-use systems and promotes the concept of automation. It produces sensors, monitors, transmitters, and standard process control systems with the ability to collect and store data, as well as customized solutions. PendoTECH staff have many decades of experience in product development, project management, embedded software development, graphical-interface programs, and mechanical engineering.
Biopharmaceutical manufacturers use sensors primarily to measure pressure, temperature, conductivity, absorbance, turbidity, and flow with applications in both upstream and downstream processing. When it comes to single-use sensors, they have questions: e.g., “Will it self-destruct?” In this case, disposabiliy is related to low cost. The single-use component is minimized by design so that most of the electronics can be recycled...
Josh Hays (upstream technology director, MilliporeSigma), BPI Theater @ INTERPHEX, April 26, 2016, 1:20–1:40 pm
Over the past 10 years, upstream processing has undergone a considerable evolution. Single-use technologies up to the 2,000-L scale have become the “gold standard” to deliver a fast, flexible, and cost-effective solution for production of recombinant proteins in a current good manufacturing practice (CGMP) environment. Cell culture media advances through speed and flexibility enhancements as raw-material modifications (along with supply continuity and reduced overall cost of operation) have also been paramount in shaping how manufacturing will look as the industry moves forward.
Although speed and flexibility in manufacturing are important, bioprocessors face a growing need to mitigate risk. This presentation by Josh Hays offered examples about how single-use upstream products are being developed with risk mitigation in mind. He examined ways that his company’s complete product and equipment por...
Peter Levison
(senior director, Pall Life Sciences ) BPI Theater @ INTERPHEX April 26, 2016, 1:40–2:00 pm
Pall’s advancements in integrated continuous bioprocessing include new product launches. The company has built a continuous laboratory at its facility in Westborough, MA. It is 6 m × 6 m and can produce >2 g of pure monoclonal antibody (MAb) per hour. One continuous process flows through one step to the next, from cell culture to formulation.
Continuous manufacturing is being used successfully in other industries; however, it is not yet used much in the biopharmaceutical industry. Regulatory authorities are now actively supporting continuous biomanufacturing. Unit operations are well established. Levison says, “A continuous process is evolutionary, not revolutionary.”
One advantage to continuous processing is making a lean, efficient system that eliminates much waste from batch processing (e.g., defects, overproduction, used talent, motion, transportation, waiting, inventory, and extra processing). O...
Bill Hartzel
(director of strategic execution, Catalent Pharma Solutions) BPI Theater at INTERPHEX, April 27, 2016 11:00–11:20 am
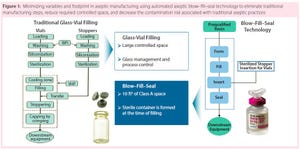
How do biopharmaceutical companies leverage technology to provide unique primary container– closures to the market space? Catalent uses an advanced aseptic process of blow–fill–seal (BFS) technology. Adding automation makes for an efficient process that can reduce or eliminate human intervention needed in critical drug-product filling applications. Catalent’s system uses one automated machine in which a container is quickly formed, filled, and sealed within the confines of the same machine. The biggest advantage of this plastic-based system over glass vials is flexibility in the design of closures. Some unique example designs include oral closures for pediatric medicines, respiratory misters, and eye droppers.
A BFS Process:
Raw plastic resin is melted at a high temperature, then formed into a molded tube (the primary container). In the first stage, the bottom of that contain...
Eduard Noguera
(managing director, Grifols) BPI Theater @ INTERPHEX, April 27, 2016, 11:20–11:40 am
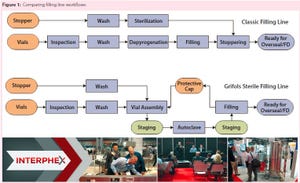
Hematologist Dr. Jose Antonio Grifols pioneered blood banking and transfusions and founded the Grifols company in Spain, 1940. Present-day Grifols specializes in plasma and protein therapies. Grifols Engineering focuses on design and equipment for process engineering. The speaker has worked for this company for about 15 years in process engineering, including fill-finish processes. He described the company’s invention — the Grifols filling method — which prevents particulate and microbial contamination during aseptic filling operations.
During this filling process, all machines are linked together to limit exposure of drug products to the environment. In step one, the first machine inspects glass vials for imperfections, then washes and dries them, assembles the vials and stoppers together, and encases them in protectors to prevent contamination. Step two places them into an autoclave for sterilization. Ne...
James D. Vogel
(founder and director, The BioProcess Institute) BPI Theater @ INTERPHEX, April 27, 2016, 11:40 am–12:00 pm
James Vogel introduced the concept of trending and how to use it to improve a manufacturing facility’s performance.
Trending
is defined as collecting data and then examining that information for trends — for example, modeling data to forecast the weather. Sometime a simple graph tells you as much as more advanced statistics can. The key is to pay attention to present conditions.
All facilities collect data to meet regulatory requirements, but doing so is worthless if no one looks at the information. By using data, companies can get an idea of what is going on in different sections of their facilities. They also can look at different facilities and determine why the same processes do not perform in the same way. Trending can illuminate systems interactions such as cleaning and environmental monitoring (even a change in cleaning staff or contracts can make a difference in environment...
A BPI Theater Roundtable at Interphex 2016
On Wednesday, 27 April 2016,
BioProcess International
and the Biomanufacturing Training and Education Center (BTEC) at North Carolina State University (Raleigh, NC) presented an afternoon training symposium in the BPI Theater at Interphex 2016.
Brian Caine, BPI’s cofounder and publisher, offered some opening remarks: “The purpose of these theaters and
BioProcess International
magazine is to talk about trends and their combined impact on bioprocessing. With the tremendous amount of new technology and products, training is imperative for companies wanting to efficiently use new technologies.”
Next, Gary Gilleskie (director of BTEC operations) offered an introduction: “Why do professionals need training and education? New technologies are driving this need. Good business results demand outstanding performance by staff. You want staff who are motivated, smart, and flexible. Those people should be good interpersonal communicators with technical knowledge and skill...
A BPI Theater Roundtable at the 2016 BIO Convention
On Tuesday, 7 June 2016, Patricia Seymour (senior consultant in process development at Bio Process Technology Consultants) chaired a lunchtime roundtable titled, “Emerging Bio-Therapies and Their Manufacturing Challenges.” She brought together a panel of three industry experts:
Mark
A
ngel
i
no (BlueB
i
rd B
i
o
t
echnology)
BlueBird is a six-year-old company specializing in gene therapy. It uses a viral vector and patients’ own cells. After processing, the patients receive their modified cells back as a curative therapy. BlueBird currently has four products in clinical trials and is providing treatments for central nervous system (CNS) disorders, thalassemia, sickle-cell disease, and cancers. The company also added a gene editing platform four years ago.
Andreas Weiler (Lonza)
Lonza has capabilities across the breadth of emerging technologies including antibodies and cell/gene therapies. The company has been in biotechnology for 30 years, and a decad...
A BPI Theater Roundtable at the 2016 BIO Convention
On Tuesday, 7 June 2016, Mike Ward (global director of content at Informa) chaired an afternoon roundtable titled, “Challenges Associated with the Industrialization and Commercialization of Gene and Cell Therapies.” He brought together a panel of four experts:
D
i
scuss
i
on
The moderator posed several questions to his panelists. “Millions of dollars are being spent on [development of] these new therapies,” he prefaced.
What challenges has the industry overcome, and which remain: commercial, technological, or regulatory? What is different in 2016 than in 1996, when we saw companies spending millions of dollars to get into gene therapy? A number of things are different in terms of gene and cell therapies and tissue engineering. We have made much progress in safety, scalability, and commercialization. In terms of science, we know more about how to express and deliver genes now. One major improvement has come with pluripotent stem cells (adult cells that ar...
Andreas Weiler
(global business unit head for emerging technologies, Lonza), BPI Theater @ BIO, June 7, 2016, 11:00–11:20 am
How do we know what the future will look like? If we look at preclinical products in the drug development pipeline, we know what is likely to come to the market over the next 5–10 years. We don’t know exactly which products will succeed, but we know the kinds of products that will be available. And a company such as Lonza always wants to prepare itself to manufacture those drugs.
Lonza was founded 120 years ago in the beautiful Swiss Alps. The company has two divisions — life sciences and pharmaceuticals — and one of its five business units focuses on emerging, personalized therapies. Lonza has 10,000 employees, US$4 billion in market capitalization, and 450 patent families (mainly related to process innovations). In 2015, the global population spent a trillion US dollars on prescription drugs, which is expected to rise by $70 million every year up to $1.4 trillion for prescription...
Jozef Orpiszewski
(senior director of program design, Fujifilm Diosynth Biotechnology), BPI Theater @ BIO, June 7, 2016, 11:20–11:40 am
Fujifilm Diosynth Biotechnology is focused on accelerating bioprocesses from gene to finished products by shortening the time line, all while following quality by design (QbD) precepts and making processes as robust as possible. When this CMO started several years ago, the development time line was about 18–24 months. Using new tools, approaches, and innovations, the company has shortened that time to 15 months or less. It can assist with project management, risk management, and mitigation as well. Using a rational program design, the company works with clients to define definite goals and time lines.
Fujifilm Diosynth Biotechnology is a global company with three sites: two in the United States (North Carolina and Texas) and one in northern England. It has introduced six commercial products. Cell culture capabilities range from small scale up to 2,000-L bioreactors.
When...
John Moscariello
(vice president of process development,CMC Biologics) BPI Theater @ BIO, June 7, 2016, 11:40 am – 12:00 pm
Most recent innovations improving efficiency have been on the upstream side of biomanufacturing. On the downstream side, bottlenecks usually occur at the chromatography step. Chromatography is such a powerful tool in purification that bioprocessors simply cannot do without it. CMC Biologics is introducing multicolumn chromatography (MCC) to help solve this bottleneck.
Batch chromatography involves a number of steps — equilibrate a column, load it, wash it, elute it, and clean it — and each step takes time. Traditionally, companies underload columns to prevent losing too much valuable product in purification. In MCC, two columns are connected, with the second column following the primary to capture product that might escape it. Once the first column is loaded, technicians can take it out of line and process it while connecting a third column to the second column and continuing chroma...
Julien Meissonnier
(vice president of science and technology, Catalent), BPI Theater @ BIO, June 7, 2016, 2:00–2:20 pm
The Catalent Applied Drug Delivery Institute (CADDI) is an organization that aims to improve comfort for patients and expand the use of advanced noninvasive delivery technologies. The institute was developed in 2012, when Catalent recognized a gap existing between academia and industry when it came to the science of noninvasive delivery. Through it, the company strives to foster the development of new technologies, share knowledge, and promote collaboration among industry leaders, academic experts, customers, and regulators.
The CADDI supports the Noninvasive Macromolecule Delivery Consortium (NMDC), which consists of a group of experts who gather regularly to share knowledge about noninvasive delivery of macromolecules (proteins, peptides, and vaccines). The consortium has four working groups that each consider a different form of noninvasive delivery: oral, pulmonary/nasal, ocular, and...
Daniel Smith
(
chief scientific officer, Cobra Biologics
) BPI Theater @ BIO, June 7, 2016, 1:40–2:00 pm
Cobra Biologics has been active in gene therapies for 20 years. The company began by manufacturing plasmid DNA for use in gene therapy products. It rapidly moved into contract manufacturing and now has a broad offering of protein virus DNA, fill–finish services, wholesale vaccines, and biologics. Based in Europe, Cobra has a facility in the United Kingdom specializing in microbial plasmid production and viral vectors for gene therapy. In Sweden, one facility focuses on mammalian antibody production and another performs large-scale microbial production with fill–finish capacity.
Recently Novartis announced 100% remission in a clinical trial using chimeric antigen receptor T (CAR-T) cells. The first such trial was performed in 2011, and it demonstrated that CAR-T-cells can treat patients with late-stage disease. Multiple trials are now under way in oncologic applications all over the world.
Cobra focuse...
Nathaniel Hentz
(assistant director of the analytical laboratory at BTEC) BPI Theater @ BIO, June 7, 2016, 2:20–2:40 pm
The Biomanufacturing Training and Education Center (BTEC) is part of the College of Engineering at North Carolina State University. In addition to training students and employees in the bioprocess industry, it also provides training for investigators with the US Food and Drug Administration (FDA). BTEC can demonstrate an entire process through upstream production, downstream processing, and drug-product filling in its manufacturing training facility. The center can train students on bioprocess analyticals as well.
For the past eight years, BTEC has worked with the FDA in developing a program that trains investigators to work in the biopharmaceutical realm. Many investigators are more experienced with small molecules and food processing. The BTEC training helps them understand present-day biopharmaceuticals.
BTEC breaks the bioprocess up into four courses: quality control (QC) analysis, ...
Ruta Waghmare
(director of worldwide emerging biotech market sales development in global pharma processing, Millipore Sigma), BPI Theater @ BIO, June 8, 2016 11:00–11:20 am
Millipore Sigma recently surveyed emerging biotechnology companies, defining those as companies with US$500 million or less in annual revenues. Such companies reported that one of their greatest challenges is attracting investors despite a rise in venture financing. In North America, the amount of funds involved in each financing round has increased, but the number of deals has stayed consistent. In Asia, both have been going up. It can be difficult for small players to obtain financing because of the increasing number of companies operating and preclinical molecules being developed. Between 2014 and 2015, the number of preclinical molecules in development worldwide increased by 17%.
However, 82% of emerging biotech companies remain optimistic. They are planning to add production and development capacity. When asked to describe their ...
Hanns-Christian Mahler
(head of drug product services, Lonza), BPI Theater @ BIO, June 8, 2016 11:20–11:40 am
A drug product transfers a biomolecule (drug substance) into a form that will be usable by patients. It contains the active ingredient with excipients and can include packaging or a device for administration. In many cases, patients must be able to administer drugs to themselves. “Think about how the drug will be administered before designing your formulation,” Mahler suggested.
Keeping a biomolecule intact is a challenge during both manufacturing and integration of drug and application device. Other challenges encountered during development might include protein stability, protein aggregation, and particle formation. Companies must find primary packaging that will not interact with their formulations.
High-concentration formulations are necessary in cases such as injections to the eye, a location where only small volumes can be used without increasing eye pressure. High concentrations are especi...
Greg Adams
(director of analytical and formulation development, FUJIFILM Diosynth Biotechnologies), BPI Theater @ BIO, June 8, 2016 11:40 am –12:00 pm
Fujifilm Diosynth Biotechnologies is a global contract development and manufacturing organization (CDMO) that has worked on multiple molecules. Adams said that proteins are complicated molecules with three dimensional (3-D) structures (primary, secondary, and tertiary) that are apt to self-aggregate. Monoclonal antibodies (MAbs), which we think of as relatively simple proteins, actually have very complex 3-D structures, with multiple chains linked by disulfide bonds, and they can be divided into multiple subclasses. A host of analytics are required to describe the function of any given protein.
Fujifilm Diosynth’s analytical philosophy is driven by understanding each product’s critical quality attributes (CQAs). The company wants to be able to analyze a product consistently all the way through good manufacturing practice (GMP) manufacturing. Its analytics ...
Michael Merges
(director of Catalent Biologics), BPI Theater @ BIO, June 8, 2016, 1:40–2:00 pm
Many small companies do not have the expertise or specialized technologies to do the necessary testing that underlies biologics manufacturing. Expertise is needed in microbiology, raw materials testing, characterization, and method development. At the same time, testing is taking place earlier in product development. Bioassays are the most outsourced service, and that is expected to continue.
Catalent has over 25 years of experience in characterizing and testing biologics. The company employs more than 100 degreed scientists and offers a broad range of biologic analytical capabilities. Catalent can provide method development at any phase, method optimization and transfer, reference standard characterization, and phase-specific validation of all methods. It can provide all necessary testing as well.
Merges presented a case study of a bioassay development project involving a high-throughput process. The product w...
Greg Beattie
(corporate vice president for global insourcing solutions and research support services, Charles River Laboratories), BPI Theater @ BIO June 8, 2016, 2:00–2:20 pm
When you think of Charles River Laboratories,” Beattie began by asking, “do you associate it with anything other than rodents?” The company is very well known for selling rats and mice. However, it is also one of the world’s largest outsourcers in the biological testing field. The company is also one of the largest “insourcing” companies: It has 800 employees spread over 70 different contracts in Europe and North America.
Insourcing
is an alternative to outsourcing and a way to leverage internal capacities by bringing in a provider who can conduct testing within the client’s own physical location. Reasons to insource include maintaining internal control and oversight. An outsource can be looked at as an extension of a client’s organization, but insourcing offers direct control while providing needed expertise.
Key considerations w...
A BPI Theater Roundtable at the 2016 BIO Convention
On Wednesday, 8 June 2016, Gil Roth (president of the Pharma and Biopharma Outsourcing Association, PBOA) chaired a lunchtime roundtable titled, “Making the Correct Outsourcing Decisions.” He brought together a panel of four experts:
Introductions
Roth began by describing his nonprofit trade group. PBOA represents contract manufacturing organizations (CMOs) and contract development and manufacturing organizations (CDMOs) in working with the United States Congress and the US Food and Drug Administration (FDA), as well as on issues within the outsourcing industry.
Lewis said that Cook Pharmica (located in Indiana) provides contract manufacturing services for biologics manufacturing as well as vial and syringe filling. The company offers a full range of packaging and medical device solutions, as well.
Sanford said that Catalent works on bulk manufacturing of large molecules. He also talked about the GPEx platform for cell line development.
Powell said that ...
A BPI Theater Roundtable at the 2016 BIO Convention
On Wednesday, 8 June 2016, Joshua Speidel (managing director of the commercial practice team at the Latham Biopharm Group) chaired an afternoon roundtable titled, “On- Demand Product Development Teams.” He brought together a panel of four experts:
Introductions
Speidel said the Latham Group works with a small companies that need external resources. “We help them gain needed resources through outsourcing and forming relationships.” For this roundtable, he wanted to find out from senior executives when they choose to bring in external resources, what those resources are, how they manage them using best practices.
Linna has experience developing both drugs and vaccines. Her consultancy supporting the drug development needs of clients in those fields.
Jojorian said that he works with Patheon customers in transferring their assets from development laboratories to manufacturing. His background is primarily in process development. He wanted to provide some insi...