January-February 2022 Featured Report
ON THE COVER: Artist’s rendering of heavy-chain–only antibodies with variable domains in light purple and conventional immunoglobulins with variable domains in light green.
(HTTPS://STOCK.ADOBE.COM)
Monoclonal antibodies (MAbs) remain the dominant biopharmaceutical product class, but as biotechnologies have advanced in recent decades, developers have found ways to exploit their “magic-bullet” capabilities while putting aside their limitations. That has led to a new generation of antibody-related therapeutics created by cutting and pasting molecular domains. Researchers are mixing and matching functional moieties of antibodies and other molecules to create custom-designed proteins with powerful efficacy and tunable targeting. A simple search at Taylor & Francis Online (
https://www.tandfonline.com
), the academic journal portal of BPI’s parent company, turns up hundreds of citations involving antibody fragments, conjugates, and bi-/multispecific molecules from the past year alone. Many articles, some of w...
Glycosylated bispecific immunoglobulin antibodies — heavy chains in green and pink and light chains in blue and yellow — engineered to target two different antigens.
(HTTPS://STOCK.ADOBE.COM)
To meet the ongoing need for new and improved drugs, the biopharmaceutical community strives to create molecules with new functions. Bispecific antibodies (bsAbs), which can simultaneously home in on two different targets, illustrate the scientific ingenuity needed for this task. The basic proof of concept for these complex molecules was established in 1960 (
1
), and their application to the redirection of effector cells was reported in the mid-1980s (
2–4
), but producing them has proved to be challenging.
Many technical advances, both large and small, were needed before bsAbs could be prepared in quantities that would be sufficient for their clinical development. Key among these advances was the invention of the single-chain variable fragment (scFv) and the creation of methods that could enable antibody protein c...
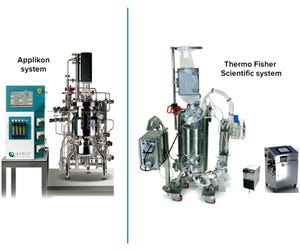
Single-use fermentors (SUFs) offer dramatic advantages over traditional stainless-steel clean-in-place (CIP)/sterilization-in-place (SIP) fermentors. Single-use technology eliminates the need for steam, chemicals, and water to clean and sterilize stainless vessels, which provides a direct reduction in capital cost and environmental-impact mitigation. Single-use platforms also increase equipment and process flexibility significantly, making it possible to switch campaigns from one product to another in minimal time, while eliminating cleaning and associated validation steps (
1
). Both Cytiva and Thermo Fisher Scientific offer SUFs that mimic the principles of historical stainless-steel systems (
2, 3
). We have evaluated SUF equipment from both vendors, reporting our evaluation of Xcellerex XDR-50 SUF and HyPerforma S.U.F. 30 units compared with a Sartorius SIP fermentor at a working volume of 10 L (
4, 5
). In that report,
Escherichia coli
batches grew to low-level optical densities (20–30 OD
600
) tha...
Early in 2021 at the American Society of Clinical Oncology’s (ASCO’s) annual meeting, attendees witnessed the first validation of a novel checkpoint target: lymphocyte activation gene 3 (LAG-3). Bristol Myers Squibb’s (BMS’s) recent success in a phase 3 study of the relatlimab anti-LAG-3 monoclonal antibody (MAb) proved that the combination of LAG-3 and programmed cell death protein 1 (PD-1) is more effective than the standard of care in first-line metastatic melanoma (
1
). For about seven years, that standard has been the same company’s Opdivo (nivolumab), a PD-1 immune-checkpoint inhibitor (ICI). This marks the first time since the registration of nivolumab and Merck’s anti-PD1 MAb Keytruda (pembrolizumab) that a new ICI has been validated in a phase 3 setting. If these findings are validated further in other applications (e.g., advanced carcinomas), then LAG-3 will become the third immune-checkpoint molecule to be targeted widely in oncology clinics — following cytotoxic T-lymphocyte–associated protei...
Subscribe to receive our monthly print or digital publication
Join our 70,000+ readers. And yes, it's completely free.