March 2019 Featured Report
On the cover: A formulator at Rentschler Biopharma (WWW.RENTSCHLER-BIOPHARMA.COM)
Quality by design (QbD), risk management, and new technologies are shaping biologics formulation work in the 21st century. We saw much evidence of this at the BioProcess International Conference and Exhibition in Boston last fall, where a wide range of talks filled the Drug Product, Fill–Finish, and Formulations track during the week after Labor Day.
Dingjiang Liu (Regeneron) offered a high-level discussion from the BioPhorum Development Group (BPDG) on “An Intercompany Perspective on Biopharmaceutical Product Robustness Studies.” Such studies ensure that drug products meet quality standards within established ranges. Liu highlighted common themes found throughout the industry, beginning with overall robustness being defined by both formulation and manufacturing processes. Integrating QbD principles, robustness is important to setting critical quality attribute (CQA) control strategies and commercial specifications. Most com...
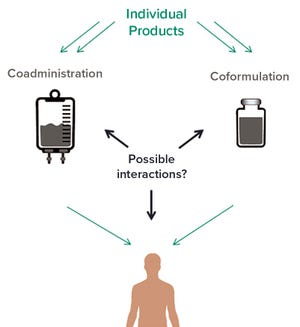
Figure 1: Comparing considerations for analytical development of coformulated products with those of coadministered products
Coformulation of two or more proteins in a single formulation is an emerging approach to delivering multiple biotherapeutics that previously have been administered in sequence. This approach brings multiple benefits to all stakeholders. Foremost for patients, the primary benefits are combined therapeutic effects and improved convenience (e.g., fewer administration events). Healthcare providers see logistical benefits and decreased risk of medical errors. Additionally, coformulations also simplify manufacturing logistics, reduce costs of packaging and distribution, and provide new opportunities for product portfolio development and marketing. However, a coformulation approach can be used only for therapeutics that share the same dosing frequency, and it reduces dosing flexibility because of the constant ratio of products in formulation.
Drug-product manufacturers have a few options f...
Early Stage Development of Advanced Formulations in the Drug Development Process Provides Competitive Advantages: Survey Predicts That Drug Product Formulation Recognition and Budgets Will Increase SignificantlyEarly Stage Development of Advanced Formulations in the Drug Development Process Provides Competitive Advantages: Survey Predicts That Drug Product Formulation Recognition and Budgets Will Increase Significantly
Figure 1: Respondents’ assessment of project failure or delay because of formulation challenges
New antibody formats and aggregate-prone, subcutaneously administered protein therapeutics present biopharmaceutical companies with major challenges regarding protein stability and aggregation. At the same time, protein stability often is not given enough attention in early stages of development. Protein aggregation reduces drug activity so that increasing doses are needed to achieve the same desired effect. Even worse, protein aggregates can induce immunogenecity that endangers patients and compromises product approval.
A market study presented for the first time at the Bio-Europe 2018 conference confirmed those challenges and the need for solutions (
1
). The study was based on the 2018 Survey, “Formulation in the Drug Product Development Process,” from Informa Pharma Intelligence, which was initiated by Rentschler Biopharma SE and Leukocare AG. The results provide current insights into the status quo and fut...
Subscribe to receive our monthly print or digital publication
Join our 70,000+ readers. And yes, it's completely free.