Opportunities in the Field of Host-Cell Proteins Part 1: Their Sources and Implications for Protein-Drug EfficacyOpportunities in the Field of Host-Cell Proteins Part 1: Their Sources and Implications for Protein-Drug Efficacy
September 28, 2022
Cathepsins and other proteases derived from host cells can cleave therapeutic proteins, diminishing product efficacy. https://www.wikipedia.com
Biopharmaceuticals are produced in genetically modified cells; thus, they contain process-and product-related impurities. Those deriving from manufacturing processes include host cell DNA/RNA, viral DNA/RNA, cellular debris, lipids, and host-cell proteins (HCPs) (1). Mammalian, bacterial, fungal, insect, and plant cell lines have been used to overexpress recombinant proteins. Currently, the most frequently used hosts for biomolecule synthesis are Escherichia coli and Chinese hamster ovary (CHO) cells.
E. coli has been used to produce heterologous proteins since the beginning of the biotechnology industry. The expression system is attractive because it grows quickly and effectively using inexpensive culture media. It also produces large amounts of protein from high-copy plasmids. Although heterologous proteins expressed in this species are not glycosylated, they still can elicit their biological responses if their amino-acid sequences are identical to those of human proteins. Thus, several different strategies can be undertaken to prevent protein-expression problems (2).
Today, CHO cells are the most frequently used expression system for heterologous proteins. Theodore Puck first cultivated such cells in 1957 (3, 4). That work initiated a new era in biotechnology. Until then, Chinese hamsters were considered to be useful only for experimental design. These cells can express proteins with glycosylation patterns that are similar enough to their human counterparts to generate the same biological activity (5).
Current purification processes cannot yield biological drug substances that are completely free of HCPs. The problem comes primarily from the complex matrices of host cells: The E. coli and CHO genomes contain ~4,288 and 25,000–30,000 protein-coding genes, respectively (6, 7). Separation and purification processes are based on a biologic’s physicochemical properties, so the possibility of HCPs to coelute alongside therapeutic proteins with similar physicochemical characteristics is significant.
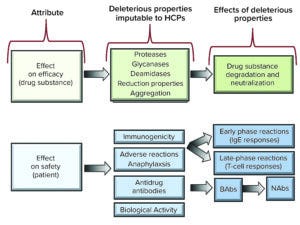
Figure 1: Effects and putative effects of host-cell proteins (HCPs) on protein-drug efficacy and safety (IgE = immunoglobulin E antibodies; BAbs = binding antibodies; NAbs = neutralizing antibodies.
Such impurities can raise concerns about drug-product efficacy and safety depending on their properties and levels. Several studies identify HCP content as a critical quality attribute (CQA) that often is used as a benchmark during biologics manufacturing (8–11). Potential risks include protease (9, 12, 13), glycosidase (14, 15), deamidase (16), and oxidation activity (17), as well as immunogenic reactions in patients (18, 19). Figure 1 characterizes putative HCP effects on efficacy and safety.
Several reviews have been compiled about HCPs. Over four installments, we will survey frequently cited articles to provide a comprehensive analysis of available data. In this first part, we summarize relevant regulatory frameworks and detail potential effects of HCPs on biologic efficacy.
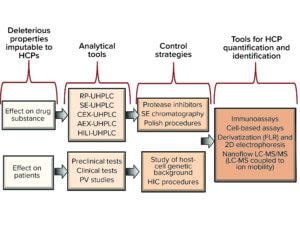
HCPs = host-cell proteins; RP = reversed phase; UHPLC = ultrahigh-performance liquid chromatography; SE = size exclusion; CEX = cation exchange; AEX = anion exchange; HILI = hydrophilic interaction; PV = pharmacovigilance; HIC = hydrophobic interaction chromatography; FLR = fluorescence; 2D = two dimensional; LC-MS/MS = liquid chromatography with tandem mass spectrometry.
Regulation: Setting Limits
Because HCPs can compromise patient safety, international guidelines have established limits and controls for such impurities (Figure 2). The US Code of Federal Regulation (CFR) reads that biologics must be “free of extraneous material except that which is unavoidable” (20). ICH Q6B <2.1.4> on Specifications: Test Procedures and Acceptance Criteria for Biotechnological/Biological Products states the following:
Process-related impurities in the drug substance may include cell culture media, host cell proteins, DNA, monoclonal antibodies, or chromatographic media used in purification, solvents, and buffer components. These impurities should be minimized by the use of appropriate, well-controlled manufacturing processes. . . .
Individual and/or collective acceptance criteria for impurities (product-related and process-related) should be set, as appropriate. Under certain circumstances, acceptance criteria for selected impurities may not be necessary (section 2.3). (21)
The World Health Organization (WHO) suggests the following:
Process validation studies should include appropriate evaluation of the process and process steps . . . and the provision of evidence that they are capable of consistently delivering quality product and intermediates. (22)
However, the same guidance notes that “routine testing may not be necessary for some impurities for which the process has been demonstrated to achieve high reduction levels.” The European Pharmacopoeia adds that HCP limits “must be approved by the competent authority” (23).
Based on such passages, it is clear that regulatory guidances have not specified allowable HCP limits, probably because such impurities are diverse, appear in low quantities in drug products, and often raise minimal immunogenicity concerns. Some studies mention 1–100 ppm as a generally acceptable range for HCPs (24–26). However, that range is not official.
Section <1132> of the United States Pharmacopeia 39–National Formulary 34 compendium lists requirements for “Residual Host Cell Protein Measurement in Biopharmaceuticals,” describing limits as “reject limits,” or measurements at which a drug product is considered to be unfit for use (27). Those limits are based on clinical data.
Effects on Efficacy
Enzymatic activities are detrimental for glycoproteins. Proteolysis, hydrolysis of glycosidic bonds, deamidation, and disulfide-bond reduction are some examples relating to HCPs.
Protease Activity: Proteases can be subdivided into serine, cysteine, aspartyl, and metalloprotease families, all of which can diminish protein-drug performance (13). Proteolytic enzymes such as pepsin and papain often target unstructured hinge regions of proteins and antibodies. Because upstream and downstream processes generate myriad proteases, selection of suitable protease inhibitors is a common practice in the biopharmaceutical industry. Such inhibitors are classified by size: e.g., small molecules (phenylmethylsulfonyl fluoride), peptides (E-64), and proteins (aprotinin) (28).
Gao et al. have identified pepsin-related proteolytic activity in an IgG1 monoclonal antibody (MAb) drug substance (12). The team writes that material had been purified using protein A affinity chromatography and orthogonal polishing steps. Samples were incubated at 40 °C for 20 days at pH 4–6. Pepstatin A was applied to some samples to inhibit proteolysis. Enzyme-related MAb fragmentation was measured using size-exclusion high-performance liquid chromatography (SE-HPLC) and liquid chromatography–mass spectrometry (LC–MS).
The team discovered that pepstatin A concentrations of 0.5 µg/mL prevented proteolysis in the spiked samples, and only hydrolytic activity persisted in the drug substance. Pepsin was active at pH 4, but activity decreased at pH 6. Findings were similar for hydrolytic activity, which was enhanced at pH 4 but minimized at neutral pH. It was noteworthy that MAb hydrolysis persisted even in the presence of pepstatin A at pH 6, although that activity was low.
Incubation at 40 °C for 20 days at pH 4 is an uncommon procedure for testing biotechnology-product stability. Such conditions typically are not applied during purification processes and thus are not used during stability testing — not as often as incubation at 37 °C for 15 days at pH 7, e.g. However, this “accelerated stress test” is an intelligent approach that can prevent issues with long-term product shelf life. In a lyophilized drug product at 4 °C, molecular degradation processes are slower than at 40 °C. Thus, proteolytic activity can go undetected.
A study by Robert et al. identified two bioprocess-derived proteases in drug substances of another MAb product. One of the enzymes was a homologue of cathepsin B. The other was inhibited by ethylenediaminetetraacetic acid (EDTA) and thus was a metalloprotease (13).
Researchers have outlined the importance of cell viability, pH optimization, appropriate control of culture temperature, and timely protein harvesting (14, 15, 29). Such factors highlight that cell debris diminishes the quality of recombinant proteins secreted into culture media.
Many types of proteases can be generated depending on the expression system applied: 1,436 proteases derive from E. coli, 216 from Saccharomyces cerevisiae, and 125 from Pichia pastoris (30). Such variety raises obstacles for purification of biotechnological products.
Thus, protease inhibitors frequently are used during bioprocessing, especially when recombinant proteins come from inclusion bodies (31). In such cases, residual protease activity can contaminate and/or degrade product proteins. In consequence, many inhibitors have been developed over the past 40 years, including multiple mixes that are commercially available.
Although proteases are generated during processing, they can be tracked during drug development, stability studies, and quality control analyses. However, case studies like that of Gao et al. must be taken in to account because protease activity can compromise drug-product efficacy.
Glycosidase Activity: Researchers have demonstrated β-galactosidase, hexosaminidase, and fucosidase activity at acidic pH levels (32, 33). Gooche et al. also have detected sialidase activity in CHO cell-culture supernatants at pH 7.5. Such work has revealed that glycosidase activity in supernatant can increase glycoprotein heterogeneity (15). Thus, sialidase activity has become a relevant CQA that directly affects MAb product pharmacokinetics (34).
Deamidase Activity: Lee et al. recently analyzed HCP proteomics using nanoflow LC-MS (16). Their test detected 2,145 HCPs in supernatants from fed-batch cultures of CHO cells. One identified species was legumain (Lgmn), a lysosomal protease that removes amide groups from aspartate amino acids in MAbs, thus changing their acidity profiles. Of note was that Lgmn ranked sixth among the 30 most abundant HCPs in the tested supernatants, accounting for about 2% of total HCP mass.
The researchers also detected cathepsin A, B, D, L, and Z (CTSZ), all of which cleave C-terminal heavy-chain fragments. Among those proteases, CTSZ was the most abundant (20th place among the 30 most abundant HCPs) in the tested supernatants. The team also detected two other proteins with glycosidase activity called β-galactosidase 1 (GLB1) and β-1,4-galactosyltransferase (B4GALT1). This team suggests that concentrations of the cited HCPs correlate with MAb quality attributes. CHO cells secrete large amounts of such impurities into culture media.
Disulfide-Bond Reduction: Shear forces can influence the number of impurities that are generated during manufacturing of a protein product. Trexler-Smith et al. have used nonreducing sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) to study the heavy and light chains of shear-stressed IgG1 MAbs. The researchers found significant disulfide-bond reduction. However, they determined that reducing enzymes can be prevented from forming during upstream processing and, if necessary, eliminated during protein A capture (17).
The Importance of HCP Monitoring
Our review of previous research in the field of HCPs highlights the need to consider factors relating to process performance. In the examples that we have discussed, even minute amounts of proteases, glycosidases, deamidases, and reducing enzymes were deleterious to therapeutic proteins. Fortunately, the activity of such enzymes can be followed closely using state-of-the-science analytical methods such as cation-exchange (CEX), anion-exchange (AEX), and hydrophobic-interaction (HI) chromatography methods, including application of ultrahigh-performance liquid chromatography (UHPLC) technology and/or coupling with mass spectrometry (e.g., LC-MS, UHPLC-MS).
Risks for immunogenicity, allergic reactions, antidrug antibodies (ADAs), and biological activity cannot be monitored as easily as those associated with proteases, glycosidases, deamidases, and reducing enzymes. Thus, it is important to differentiate between a protein product’s physicochemical quality attributes and immunogenic characteristics.
The next installment of our review, which will appear in BPI’s October 2022 issue, focuses on HCPs that can compromise patient safety. Part three provides case studies in HCP detection and identification. The series concludes with best practices for HCP risk management and exploration of the future of HCP analytics.
Acknowledgments
This work was supported by Mexico’s Consejo Nacional de Ciencia y Tecnología (CONACyT), Grant FINNOVA-CONACyT 174104. Authors VPMM and NOP are Sistema Nacional de Investigadores (SNI)-L1 CONACyT Fellows.
Disclosures
Probiomed S.A. de C.V. develops, manufactures, and markets biosimilar products. All three authors are involved in the development of biosimilar products for Probiomed.
References
1 Briefing Document: BLA 125545, “Epoetin Hospira”, a Proposed Biosimilar to Epogen/Procrit (Epoetin Alfa). US Food and Drug Administration, Oncological Drugs Committee: Silver Spring, MD, 2017; https://www.fda.gov/files/20170525-ODAC-02-Hospira_Backgrounder.pdf.
2 Baneyx F. Recombinant Protein Expression in Escherichia coli. Curr. Opin. Biotechnol. 10(5) 1999: 411–421; https://doi.org/10.1016/s0958-1669(99)00003-8.
3 Puck T, Kao FT. Genetics of Somatic Mammalian Cells: Section IV — Properties of Chinese Hamster Cell Mutants with Respect to the Requirement for Proline. Genetics 55 1967: 513–524; https://doi.org/10.1093/genetics/55.3.513.
4 Puck T and Kao FT. Genetics of Somatic Mammalian Cells Treatment with 5-Bromodeoxyuridine and Visible Light for Isolation of Nutritionally Deficient Mutants. PNAS USA 58 1967: 1227–1234; https://doi.org/10.1073/pnas.58.3.1227.
5 Wacker C, et al. Glycosylation Profiles of Therapeutic Antibody Pharmaceuticals. Euro. J. Pharm. Biopharm. 79(3) 2011: 503–507; https://doi.org/10.1016/j.ejpb.2011.06.010.
6 Blattner FR, et al. The Complete Genome Sequence of Escherichia coli K-12. Science 5 September 1997: 1453–1462; https://doi.org/10.1126/science.277.5331.1453.
7 Gibbs RA, et al. Genome Sequence of the Brown Norway Rat Yields Insights into Mammalian Evolution. Nature 428, 2004: 493–521; https://doi.org/10.1038/nature02426.
8 Zhu-Shimoni J, et al. Host Cell Protein Testing by ELISAs and the Use of Orthogonal Methods. Biotechnol. Bioeng. 111, 2014: 2367–2379; https://doi.org/10.1002/bit.25327.
9 Zhang Q, et al. Comprehensive Tracking of Host Cell Proteins During Monoclonal Antibody Purifications Using Mass Spectrometry. mAbs 6(3) 2014: 659–670; https://doi.org/10.4161/mabs.28120.
10 Hogwood CE, Bracewell DG, Smales CM. Host Cell Protein Dynamics in Recombinant CHO Cells: Impacts from Harvest to Purification and Beyond. Bioengineered 4(5) 2013: 288–291; https://doi.org/10.4161/bioe.23382.
11 Wang X, Hunter AK, Mozier NM. Host Cell Proteins in Biologics Development: Identification, Quantitation, and Risk Assessment. Biotechnol. Bioeng. 103(3) 2009: 446–458; https://doi.org/10.1002/bit.22304.
12 Gao SX, et al. Fragmentation of a Highly Purified Monoclonal Antibody Attributed to Residual CHO Cell Protease Activity. Biotechnol. Bioeng. 108, 2011: 977–982; https://doi.org/10.1002/bit.22982.
13 Robert F, et al. Degradation of an Fc-Fusion Recombinant Protein by Host Cell Proteases: Identification of a CHO Cathepsin D Protease. Biotechnol. Bioeng., 104, 2009: 1132–1141; https://doi.org/10.1002/bit.22494.
14 Gramer MJ. Detecting and Minimizing Glycosidase Activities That Can Hydrolyze Sugars from Cell Culture–Produced Glycoproteins. Molec. Biotechnol. 15, 2000: 69–75; https://doi.org/10.1385/MB:15:1:69.
15 Gramer MJ, Goochee CF. Glycosidase Activities in Chinese Hamster Ovary Cell Lysate and Cell Culture Supernatant. Biotechnol. Prog. 9, 1993: 366–373; https://doi.org/10.1021/bp00022a003.
16 Park JH, et al. Proteomic Analysis of Host Cell Protein Dynamics in the Culture Supernatants of Antibody-Producing CHO Cells. Sci. Rep. 10(7) 2017: 1–13; https://doi.org/10.1038/srep44246.
17 Trexler-Schmidt M, et al. Identification and Prevention of Antibody Disulfide Bond Reduction During Cell Culture Manufacturing. Biotechnol. Bioeng. 106(3) 2010: 452–461; https://doi.org/10.1002/bit.22699.
18 Wadhwa M, et al. Immunogenicity of Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF) Products in Patients Undergoing Combination Therapy with GM-CSF. Clin. Cancer Res. 5, 1999: 1353–1361; https://pubmed.ncbi.nlm.nih.gov/10389919.
19 Bierich JR. Treatment of Pituitary Dwarfism with Biosynthetic Growth Hormone. Acta Paediatr. Scand. 325, 1986: 13s–18s. https://doi.org/10.1111/j.1651-2227.1986.tb10357.x.
20 21 CFR <610.13>. General Biological Products Standards. US Fed. Reg. 3 April 2015: 18093; https://www.federalregister.gov/documents/2015/04/03/2015-07268/food-and-drug-administration-regulations-change-of-addresses-technical-amendment.
21 ICH Q6B. Specifications: Test Procedures and Acceptance Criteria for Biotechnological/Biological Products. European Medicines Agency: London, UK, September 1999; https://www.ema.europa.eu/en/documents/scientific-guideline/ich-q-6-b-test-procedures-acceptance-criteria-biotechnological/biological-products-step-5_en.pdf.
22 Annex 4: Guidelines on the Quality, Safety, and Efficacy of Biotherapeutic Protein Products Prepared by Recombinant DNA Technology. World Health Organization: Geneva, Switzerland, 18 June 2014; https://www.who.int/publications/m/item/recombinant-dna-annex-4-trs-no-987.
23 Erythropoietin. European Pharmacopoeia 9.0, Vol. 2, Germany, Monographs D–E. European Pharmacopoeia Commission: Strasbourg, France, 2016: 2309.
24 Chon JH, Zarbis-Papastoitsis G. Advances in the Production and Downstream Processing of Antibodies. New Biotechnol. 28(5) 2011: 458–463; https://doi.org./10.1016/j.nbt.2011.03.015.
25 Champion K, et al. Defining Your Product Profile and Maintaining Control
Over It, Part 2. BioProcess Int. 3(8) 2005: 52–57; https://bioprocessintl.com/2005/september-2005/defining-product-profile-maintaining-control-part-2.
26 Eaton LC. Host Cell Contaminant Protein Assay Development for Recombinant Biopharmaceuticals. J. Chromatogr. A 705(1) 1995: 105–114; https://doi.org/10.1016/0021-9673(94)01249-e.
27 General Chapter <1132>: Residual Host Cell Protein Measurement in Biopharmaceuticals. USP 39–NF 34. United States Pharmacopeia: Rockville, MD, 2016; https://doi.org/10.31003/USPNF_M8647_01_01
28 Ritchie C. Protein Purification. Mater. Meth. 2, 2012: 134; https://dx.doi.org/10.13070/mm.en.2.134.
29 Chuan KH, et al. Caspase Activation, Sialidase Release and Changes in Sialylation Pattern of Recombinant Human Erythropoietin Produced by CHO Cells in Batch and Fed-Batch Cultures. Cytotechnol. 51(2) (2006): 67–79; https://doi.org/10.1007/s10616-006-9016-5.
30 Rawlings N, et al. The MEROPS Database of Proteolytic Enzymes, Their Substrates and Inhibitors in 2017 and a Comparison with Peptidases in the PANTHER Database. Nucleic Acids Res.; 42, 2017: D503–D509; https://doi.org/10.1093%2Fnar%2Fgkt953.
31 Jürgen B, et al. Quality Control of Inclusion Bodies in Escherichia coli. Microbial Cell Factories 9, 2010: 41; https://doi.org/10.1186/1475-2859-9-41.
32 Munzert E, et al. Sialidase Activity in Culture Fluid of Chinese Hamster Ovary Cells During Batch Culture and Its Effect on Recombinant Human Antithrombin III Integrity. Biotechnol. Prog. 12, 1996: 559–563; https://doi.org/10.1021/bp9600086.
33 Sliwkowski MB, Gunson JV, Warner TG. Sialylation and Phosphorylation as a Function of Culture Conditions for Recombinant Human Deoxyribonuclease Produced by CHO Cells. J. Cell. Biochem. 16, 1992: 150.
34 Liu L. Antibody Glycosylation and Its Impact on the Pharmacokinetics and Pharmacodynamics of Monoclonal Antibodies and Fc-Fusion Proteins. J. Pharm. Sci. 104, 2015: 1866–1884; https://doi.org/10.1002/jps.24444.
Víctor Pérez Medina Martínez and Carlos Eduardo Espinosa-de la Garza work in the research and development unit, and corresponding author Néstor O. Pérez works in operations management at Probiomed S.A. de C.V., Cruce de Carreteras Acatzingo-Zumpahuacán s/n, Tenancingo, Estado de México, México. C.P. 52400; 52-55-1166-2305; [email protected]; https://www.probiomed.com.mx.
You May Also Like





