A Vaccine Case Study: Qualifying Redundant Disconnection Technologies As Container-Closure Systems for Long-Term Storage and ShippingA Vaccine Case Study: Qualifying Redundant Disconnection Technologies As Container-Closure Systems for Long-Term Storage and Shipping
 The expanding complexity of biopharmaceutical manufacturing puts increasing pressure on single-use systems to meet the demands of the modern industry’s global footprint. Individual sites within a given organization often are specialized to a fixed number of “modular” process steps (1). Such product segregation increases plant efficiency and output while making the best of staff competencies. But it also can create an additional need for transportation of intermediate or bulk drug substance (BDS) over long distances.
The expanding complexity of biopharmaceutical manufacturing puts increasing pressure on single-use systems to meet the demands of the modern industry’s global footprint. Individual sites within a given organization often are specialized to a fixed number of “modular” process steps (1). Such product segregation increases plant efficiency and output while making the best of staff competencies. But it also can create an additional need for transportation of intermediate or bulk drug substance (BDS) over long distances.
 Freezing generally is used to lengthen the shelf life of such materials for global shipping and to ensure their stability and security during transport (2, 3). But it places an additional burden on single-use systems (requiring them to withstand exposure to subzero temperatures) and on drug manufacturers (who must prove the suitability of such systems for use with their processes, storage conditions, and handling procedures). Plastics become brittle at lower temperatures, making physical handling an important consideration in single-use system design for applications that involve freezing.
Freezing generally is used to lengthen the shelf life of such materials for global shipping and to ensure their stability and security during transport (2, 3). But it places an additional burden on single-use systems (requiring them to withstand exposure to subzero temperatures) and on drug manufacturers (who must prove the suitability of such systems for use with their processes, storage conditions, and handling procedures). Plastics become brittle at lower temperatures, making physical handling an important consideration in single-use system design for applications that involve freezing.
Biopharmaceutical companies such as Pfizer use process-step-segregation and further improve their plant efficiencies by incorporating single-use platform technologies to harmonize the manufacturing and dispensing of regularly produced BDS and intermediates (4). Platform technologies play a critical role because of their extensive application. Recognizing that, Pfizer continuously improves the robustness of its platforms by identifying potential supply chain risks and building redundancies to address those.
A recent platform analysis confirmed that both the single-use system and container film for storage and shipping of BDS and process intermediates are dual sourced. However, the analysis also revealed that both suppliers’ products contain a single container–closure and disconnection system for these containers. Although heat sealing of thermoplastic tubing is a sound practice, in this case the team viewed it as a potential risk because it was the sole connection mechanism used across the platform’s design. In an effort to improve process flexibility and robustness, the team concluded that a second container closure and disconnection technology was needed.
We assessed a number of commercially available disconnection technologies. Pall Kleenpak aseptic disconnectors were not chosen because each assembly would require 15 fittings per manifold, which was price prohibitive for this process. Metal crimp collars were eliminated as an option because they have to be added at the time of manifold manufacture. Thus they could not be used on existing manifold stock and would require an engineering redesign of the assemblies.
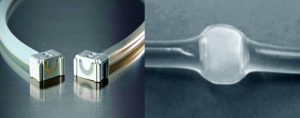
Heat sealing was the incumbent disconnection technology. But we wanted an alternative that could provide redundancy to the manufacturing process while addressing several concerns over the current methodology. Alternatives were considered if they could eliminate subjectivity from disconnection results (e.g., What does a “good seal” look like?) and offer the ability to use both thermoplastic elastomer (TPE) and silicone tubing.
However, the target temperature required for cold storage and shipping of Pfizer’s vaccine product fell outside the vendor’s product specification for Clipster connectors (5). So we performed a study to prove their suitability under the conditions of this intended use.
Purpose and Scope of the Study
Our purpose was to compare the stability of heat-sealed thermoplastic tubing and of Clipster products as a container-closure for two comparable single-use dispensing manifold assemblies during –50 ± 10 °C freezing, shipping, and room-temperature thawing of a vaccine BDS.
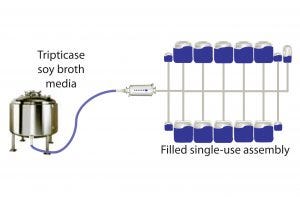
Figure 1: Closed-system dispensing
Manifolds of identical design were assembled by Sartorius Stedim Biotech SA (SSB) and Thermo Fisher Scientific (TFS) as follows:
• 50-mL, 500-mL, and 5-L Flexboy single-use containers, thermoplastic tubing, fittings, and connectors from SSB according to Figure 1
• 50-mL, 500-mL, and 5-L single-use BPC bioprocess containers from TFS with thermoplastic tubing, fittings, and connectors according to Figure 1.
Test assemblies from both manufacturers were filled with trypticase soy broth (TSB) by Pfizer manufacturing personnel according to current standard operating procedures (SOPs). TSB medium was chosen as an acceptable surrogate for process solutions in this study because of its microbial growth-promoting properties, which are necessary for container-closure integrity testing.

Table 1: Sampling description for the bag-integrity system; BCT = bacterial challenge testing
Half of the container filling lines on each manifold were closed by heat sealing; the remaining lines were closed mechanically using a Clipster connector based on the manufacturer’s recommended instructions. Table 1 describes the resulting test items produced from each test manifold along with the number of samples and specifications for passing each test.
Bacterial Challenge Testing
After dispensing, biocontainers were sealed and disconnected from the manifolds. Then each individual container was packaged in overwrap and sealed in overcartons. Thus protected, the containers were subjected to two freeze–thaw cycles, with storage at –50 ± 10 °C until frozen and thawed at room temperature. Thawed samples were frozen a third time before being shipped under temperature-controlled conditions to an independent laboratory (Nelson Laboratories LLC in Salt Lake City, UT) for the balance of testing.
 Upon arrival at the laboratory, the samples were allowed to thaw at room temperature and then inspected for system integrity based on the absence of leaks. All samples (with both container-closure types) then were incubated at 30 ± 2 °C for three days. Afterward, laboratory technicians inspected them for turbidity to ensure that they were not contaminated before performing microbial ingress experiments.
Upon arrival at the laboratory, the samples were allowed to thaw at room temperature and then inspected for system integrity based on the absence of leaks. All samples (with both container-closure types) then were incubated at 30 ± 2 °C for three days. Afterward, laboratory technicians inspected them for turbidity to ensure that they were not contaminated before performing microbial ingress experiments.

Table 2: Summary of results for test samples and positive and negative controls using Sartorius Stedim Biotech assemblies; CFU = colony-forming unit
Both macroscopic and microscopic morphology of samples from each batch of challenge organism were consistent with Brevundimonas diminuta (ATCC #19146). The sterile closure-integrity vessel was filled with B. diminuta, and the challenge solution was plated to determine microorganism titers both before and after contact with the bags.
Samples were immersed in the challenge solution and held below its surface. Approximately 20 L of solution was used for the 50-mL and 500-mL bag capacities, about 30 L for the 5-L heat-sealed bags, and about 29 L for the 5-L Clipster-sealed bags. The integrity vessel was closed, and samples were immersed in the challenge solution for no less than 30 minutes.
Then the samples were removed from the challenge solution, decontaminated with a 5% chlorine bleach solution, and rinsed with purified water. Finally, the samples were incubated at 30 ± 2 °C for seven days before visual inspection for growth of the challenge microorganism.
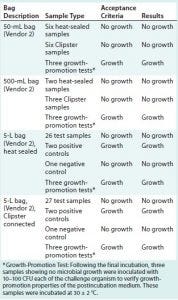
Table 3: Summary of results for test samples and positive and negative controls using Thermo Fisher Scientific assemblies; CFU = colony-forming unit
Tables 2 and 3 summarize the results for all test samples, positive and negative controls, and parallel growth-promotion tests.
Positive controls were created by piercing a single hole in a bag using a 22-gauge needle. Then all four positive controls were subjected to the same protocol as the test bags, including incubation at 30 ± 2 °C for seven days, to confirm viability of the test solution to promote microbial growth. The negative controls consisted of two bags that were not exposed to the challenge solution. They were incubated at 30 ± 2 °C for seven days to confirm the absence of contamination on those test articles before bacterial challenge by immersion.
Methods and Results
Following incubation, three nongrowth bags per group of tested samples were inoculated with 10–100 CFU of the challenge organism. Tables 4 and 5 specify the average number of colonies used for each challenge solution prepared. Tested bags were incubated at 30 ± 2 °C for a maximum of seven days or until growth was observed to confirm viability of the test solution to promote growth and thus eliminate false negatives.
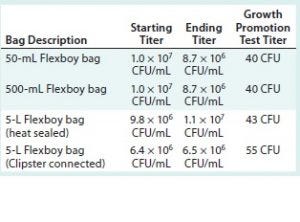
Table 4: Average number of colonies used for each challenge solution (Sartorius-Stedim Biotech bags)
All challenge-solution titers are over the specification of 1 × 106 CFU/mL at minimum. All the growth-promotion test titers fall within the specification of 10–100 CFU.
Verified Appropriate
This study was designed to compare microbial-barrier properties of the Clipster sealing and disconnection system with those of the heat-sealing method on thermoplastic tubing after filling and storage under routine conditions by Pfizer in Sanford, NC.
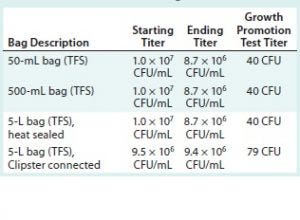
Table 5: Average number of colonies used for each challenge solution (Thermo Fisher Scientific bags)
To confirm the microbial barrier properties of bag assemblies using both closure methods, heat-sealing of thermoplastic elastomer filling tubes and mechanical closure using the Clipster system, after multiple freeze–thaw cycles and shipping, we used a bacterial challenge by immersion. All samples and controls achieved expected results described in the study protocol:
An absence of microbial growth in the negative controls verifies that bags and media were sterile before the challenge.
Microbial growth in the positive controls verifies that the test method used is suitable to demonstrate the ingress of microorganisms in those bags.
Microbial growth in the growth-promotion tests verifies that the bags are capable of promoting growth.
 After worse-case handling conditions — three freeze–thaw cycles, storage at –50 ± 10 °C, and shipping — the test samples were subjected to and passed container-closure integrity testing with a bacterial challenge by immersion. Our results confirmed that the study’s goal of validating a redundant container-closure platform for critical vaccine-intermediate, BDS, and drug-product dispensing manifolds had been met.
After worse-case handling conditions — three freeze–thaw cycles, storage at –50 ± 10 °C, and shipping — the test samples were subjected to and passed container-closure integrity testing with a bacterial challenge by immersion. Our results confirmed that the study’s goal of validating a redundant container-closure platform for critical vaccine-intermediate, BDS, and drug-product dispensing manifolds had been met.
References
1 Langer E, Lupis J-C. BioPharma Manufacturing’s Top 15 Trends in 2015. Pharmaceut. Manufac. 26 August 2015: www.pharmamanufacturing.com/articles/2015/biopharma-manufacturing-top-15-trends-2015.
2 Lipowicz M, Basta N. 2014 Biopharma Cold-Chain Forecast. Pharmaceut. Commerce 29 April 2014: http://pharmaceuticalcommerce.com/supply-chain-logistics/2014-biopharma-cold-chain-forecast.
3 Goldstein A, et al. Freeze Bulk Bags: A Case Study in Disposables Implementation. BioPharm Int. supplement, November 2009.
4 Trotter AM. Adoption of Single-Use Disposable Technology in Biopharma Industries: Manufacturing, Economic and Regulatory Issues to Consider. Amer. Pharmaceut. Rev. 15(2) 2012: 50–69.
5 SLO5704-e11011. Validation Guide: Clipster® Aseptic Disconnector. Sartorius Stedim Biotech SA: Aubagne, France, January 2011; www.sartorius.com/_ui/images/h3b/hcb/8855194402846.pdf.
Corresponding author Christine Megarity is a senior scientist at Pfizer specializing in dispensing, storage, and shipping of drug substances and intermediates. Bobbi Allen is a marketing manager at Sartorius Stedim Biotech with 15 years of focus on single-use biopharmaceutical manufacturing systems. Clipster and Flexboy are registered trademarks of Sartorius Stedim Biotech SA. Kleenpak is a registered trademark of Pall Corporation.
You May Also Like






