E. coli Cultivation in a 12L and 120L CELL-tainer™ Single-Use BioreactorE. coli Cultivation in a 12L and 120L CELL-tainer™ Single-Use Bioreactor

Although single-use bioreactors are widely applied in the biopharmaceutical industry, microbial cultivation in them is restricted due to limitations in oxygen mass transfer, mixing, and heat transfer. The CELL-tainer™ single-use bioreactor makes use of an innovative principle applying a two-dimensional (2D) rocking motion, thus providing excellent mass transfer, making this single-use reactor very suitable for microbial cultivation at different scales. With a simple pillow-shaped bag without moving parts, the technology is robust and leads to cost savings compared with other single-use or classical glass or stirred bioreactors.
Mass Transfer
In a CELL-tainer single-use bioreactor, a pillow-shaped bag is positioned in a tray. The movement of this tray is in two directions: a vertical rocking motion combined with a horizontal translation. This combination of movement results in oxygen mass transfer coefficients, kLa > 300 (h−1) both at 12L and 120L scales (working volumes). The oxygen mass transfer coefficients have been determined by using the dynamic method (Figure 1).
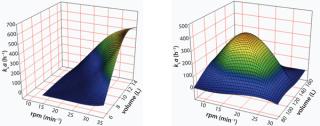
Figure 1: ()
With aeration by air, an oxygen mass transfer (OTR) of at least 100 (mmolh−1) can be achieved. As a function of the rocking speed, mixing times have been measured to be 12–16 seconds at a working volume of 12L in the 20L CELL-tainer and 60–120 seconds at a working volume of 115L in the 200L CELL-tainer.

Figure 2: ()
Scale-Up of the Cell-tainer Single-Use Bioreactor
In order to keep the fluid characteristics the same at different scales, the ratio of rocking angle (including horizontal displacement) and the length of the bag have been kept comparable at different scales such that the oxygen mass transfer coefficient will be in the same range at both scales (Figure 1). It is obvious that the kLa is strongly dependent on the liquid volume, as the kLa is a volume dependent parameter. Due to the principle of surface aeration applied and as the liquid depth also changes with volume, the shape of the wave is strongly influenced by filling volume, resulting in a volume dependent entrapment of air into the liquid.
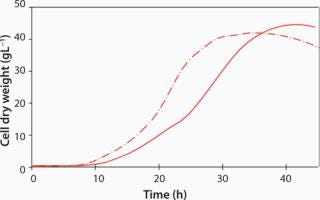
Figure 3: ()
Escherichia Coli Cultivation
In the biopharmaceutical industry, E. coli is an established microbial host organism for protein expression. The growth of E. coli BL21-Gold (Agilent Technologies Inc. USA) encoded for a maltogenic amylase has been studied at both 12L and 120Lscale in the two sizes of CELL-tainer bioreactors. Growth was conducted at a pH of 7.0 and at 37 °C. In the 12L CELL-tainer, an exponential growth during 21 h was observed up to a cell dry weight of 10 gL−1. By a controlled glucose feed, starting at 11 h, a cell dry weight of 43 gL−1 (OD600 = 140) is obtained in 32 h. Temperature was slowly decreased to 30 °C before induction. At a tenfold scale in a prototype CELL-tainer, exponential growth was observed until 29 h up to a final cell dry weight of 45 gL−1 in 39 h, which illustrates the proper scalability of the CELL-tainer technology.
GMP Application
Results from the measurement of mass transfer coefficient, kLa and E. coli cultivation show the applicability of the CELL-tainer single-use bioreactor for microbial cultivation. The advantages of single-use technology in upstream processing such as reduced efforts for validation, better sterility control, and simplified operation can now be applied to microbial processes as well. By implementing the CELL-tainer single-use bioreactor, significant cost reduction can be achieved. Moreover, reduction of time needed to implement a bioreactor in a GMP-environment can be achieved. A full site acceptance test, including installation and operational qualification can be done within two weeks after delivery of the equipment. This all contributes to significant cost savings and faster time to market.
Conclusion
The limitations in oxygen mass transfer and mixing hampered application of single-use technologies in microbial processes. As industry is widely applying microbial processes for biopharmaceutical products, there is a need for single-use microbial bioreactors as well. The CELL-tainer single-use bioreactor can support cultivations from 5 up to 120L working volume for microbial application and up to over 200L working volume for mammalian cell culture in one and the same bag.
Charter Medical, Ltd. exclusively distributes the CELL-tainer bioreactor in the United States and Europe. For further information visit www.chartermedical.com or www.celltainer.com.
About the Author
Author Details
Dr. Nico Oosterhuis is chief technical and scientific officer of CELLution Biotech BV, Assen, The Netherlands, which developed the CELL-tainer single-use bioreactor. Dr. Stefan Junne is group leader for process and systems biotechnology at the chair of bioprocess engineering Institute of Biotechnology at the Technische Universitaet Berlin.
REFERENC
ES
1.) Junne,. 2013. Cultivation of Cells and Microorganisms in Wave- Mixed Disposable Bag Bioreators at Different Scales. Chemie Ingenieur. Technik. 85:57-66.
You May Also Like






