Eight Questions to Ask Before Planning Your Single-Use Biomanufacturing FacilityEight Questions to Ask Before Planning Your Single-Use Biomanufacturing Facility

Figure 1:
Deployment of new GMP biomanufacturing capacity is a battle of tradeoffs (Figure 1). Push to accelerate your timetable? Cost or quality considerations must be compromised. Need to drive down total investment? Build-out time or quality must be sacrificed. As single-use technology establishes wider acceptance, certain tradeoffs may be lessened, but serious challenges remain.
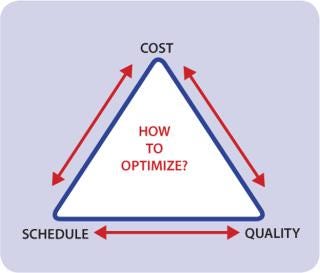
Figure 1: ()
Overcoming the limitations intrinsic to conventional biomanufacturing strategies requires a fundamental rethinking of bioproduction architecture. With the FlexFactory® biomanufacturing platform and a deeply experienced team to support its effective implementation, Xcellerex offers a new level of freedom from historical limitations.
In this paper, we examine eight critical considerations facing the biopharmaceutical and vaccine production landscape, and how each is addressed with this new, proven approach to biomanufacturing.
Question #1: Can you achieve a level of process productivity suitable for commercial success?
As the biopharmaceutical and vaccine industries race to embrace single-use technologies, questions remain as to whether process productivity and scalability issues will slow the technology’s adoption for commercial production. Increasingly, single-use performance limitations are being overcome, but certain process areas still need conversion.
FlexFactory is an open, technology-neutral platform, which means that operators can select the best combination of upstream and downstream technologies for their particular process, and Xcellerex will integrate those choices into a FlexFactory facility. Xcellerex searches out and tests nearly every emerging single-use technology to ensure the platform can accommodate the best possible configurations for client productivity. This testing — and its internal use — also enables Xcellerex to provide deep process support for a wide variety of technologies. The company’s PDMax™ process development team works with clients to apply the best available technologies and other tools to ensure that a commercially viable process is secured.
Xcellerex has successfully manufactured nearly 20 biotherapeutics and vaccines for use in clinical trials over the past several years. The company’s biomanufacturing and process development teams have accumulated an extensive body of data demonstrating that FlexFactory delivers comparable productivity and scalability across a range of cell lines, even in complex processes. Externally, Xcellerex is delivering FlexFactory technology for clinical manufacturing to leading biotherapeutics and vaccine manufacturers. Likewise, multiple 2,000-L XDR single-use bioreactors are being implemented in a commercial plant currently under construction.
(Note: Extensive productivity and scalability data on FlexFactory and XDR is available by contacting Xcellerex.)
Question #2: How long will it take to get a GMP facility built, validated and operational?
Construction, build-out, and start-up of conventional stainless steel plants could take four to five years or more, including many months of painful validation. Through the elimination of clean-in-place/steam-in-place (CIP/SIP) infrastructure, conventionally designed single-use facilities promise to cut that time by as much as half (Figure 2). The FlexFactory architecture is nearly 100% single use, thus eliminating the need for sterilization and CIP equipment.
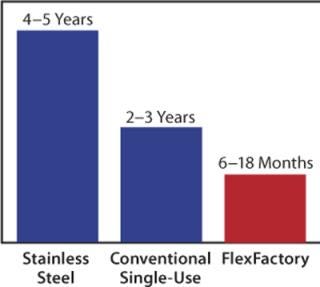
Figure 2: ()
In addition, FlexFactory takes facility simplification several steps further by using Controlled Environmental Modules (CEMs) to effectively shrink a cleanroom around each unit operation, eliminating the need for cleanroom construction, complex HVAC design, and validation. The entire upstream and downstream operation can be co-located in a simplified single-suite facility with basic HVAC and utilities requirements.
Further, Xcellerex’s turn-key process automation and electronic batch control package reduces development time and expense for the client.
Additionally, through its TransPlant™ service, Xcellerex assembles the FlexFactory line; develops standard operating procedures (SOPs); and performs system installation and operational qualification (IOQ), and operator training before shipping equipment to a customer’s facility. Once installed there, the first GMP run is typically just three to four weeks away.
The result is FlexFactory deployment time of six to 18 months. As an example, one current project will deliver a validated FlexFactory for clinical trials to a client in Europe in seven months, which includes a GMP run performed by a newly trained customer team at the Xcellerex facility.
Question #3: What will it really cost?
The move to single-use manufacturing saves considerable capital expense through the elimination of CIP/SIP infrastructure. However, single-use technology deployed in a traditional biomanufacturing architecture still requires the construction of multiple clean-room suites, as well as expensive heating, ventilation, and air conditioning (HVAC) systems for each. FlexFactory lines are housed in a single Class 300,000 suite, with a single HVAC system. The plant footprint is reduced by 40% or more (Figure 3). In total, these factors result in a capital cost for FlexFactory that is as much as 65% less than a comparable fixed-pipe stainless steel facility.
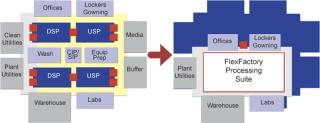
Figure 3: ()
Similarly, FlexFactory enjoys all the operating cost advantages delivered by single-use technology, including reduction in CIP/SIP cycles, validation, and turnaround times. FlexFactory furthers those gains by drastically simplifying the validation process for the cleanroom suites and HVAC systems. FlexFactory adds another layer of efficiency by allowing a single team of operators to work together in a single upstream/downstream suite. This reduces labor costs and improves communication and other efficiencies. Maintenance staff can even work within the suite because all FlexFactory mechanicals are external to the controlled environment modules. In addition, because FlexFactory can accommodate simultaneous multidrug production, utilization rates are improved, reducing cost of goods sold by 30–40%.
Question #4: What will it take to make your single-use process GMP compliant?
An important attraction of single-use technology is the reduced risk of cross-contamination by using disposable containers, which translates to improved manufacturing quality. However, multiple cleanrooms still require time-consuming validation. In addition, each piece of equipment needs to be validated, as well as the overall process.
With FlexFactory, Xcellerex delivers a complete system in “prevalidated” or validatable form. Because FlexFactory does not require large conventional cleanrooms with multiple HVAC systems, validation is simplified and accelerated.
FlexFactory also includes the eFactory process automation platform, which integrates documentation, process verification, data collection and overall control of the plant. Xcellerex trains operators to ensure they are in compliance, and eFactory ensures that each operator is qualified to execute each process step.
Question #5: How will you integrate robust process control architecture?
A commercial process requires the integration of complex upstream and downstream unit operations to work efficiently, but it also demands robust process automation architecture to ensure operational integrity and GMP compliance. When assembling a production line composed of equipment from multiple vendors, this complex task falls on the shoulders of each client’s process automation team (if one exists), or it is outsourced to an engineering firm. This can add months of work and significant additional cost to a new deployment.
Process automation can be greatly simplified because a FlexFactory plant itself is greatly simplified compared with a large, complex traditional plant. In addition, Xcellerex designs each FlexFactory with eFactory™ Process Automation. This architecture delivers true fault-tolerant redundancy across every aspect of the communications, process automation controllers (PAC), data historian, and batch execution servers.
The Xcellerex platform also includes automatically generated electronic batch records with an electronic “watch-dog.” This watch-dog reduces the chance for human error by identifying and communicating deviations in real-time, often soon enough to salvage a batch. Batches can be released by exception, eliminating lengthy paper-based quality assurance (QA) reviews and speeding up overall batch release time by weeks or months.
Question #6: Where will you get in-depth process support and training?
As noted previously, integrating a process with several emerging technologies can be a daunting challenge. Many equipment manufacturers offer very limited process expertise to support the adoption of their products, leaving a drug-maker to his own devices. This can lengthen the deployment timeline and introduce unnecessary risks and costs.
Rather than simply offering FlexFactory hardware to its clients, Xcellerex offers a comprehensive, fully integrated hardware–software–compliance–process support package. The Xcellerex team has decades of experience in biomanufacturing, Process development, systems engineering and process automation. These resources work hand-in-hand with customers throughout the planning and deployment of a FlexFactory system. In addition, Xcellerex is able to operate the FlexFactory line at its biomanufacturing operation before final deployment at a client’s facility. This provides opportunity for SOP development, system validation, and operator training. When a FlexFactory is installed at the customer location, it can be quickly site-validated and started-up by pretrained operators.
Question #7: How easy will it be to change the process or adapt it for multi-drug production in the future?
FlexFactory is perfectly tailored for simultaneous multidrug production in a single open suite. Because each unit operation is contained in its own cleanroom environment, each FlexFactory module is capable of processing a different drug at the same time. Single-use plants in a conventional manufacturing architecture are limited to producing one drug at a time in separated cleanrooms. Change-overs are simpler than with traditional stainless steel systems, but they are much more involved than what can be accomplished with FlexFactory.
Question #8: What will the US FDA and other regulators think about your disposables-based process?
Generally speaking, regulators favor single-use manufacturing because of its reduced likelihood of contamination and cross-contamination. FlexFactory offers two additional levels of safety which regulators find very appealing. First, the CEM modules place the operator outside of the cleanroom, eliminating the largest single source of potential contamination. Second, FlexFactory’s real-time electronic quality assurance monitoring of the facility provides a significantly enhanced level of compliance.
Over the course of several review meetings with the FDA, the EMEA and other regulatory bodies, FlexFactory has received positive reviews, especially around its “quality-by-design” engineering.
Conclusion
The shift to single-use technology brings exciting advantages to biopharmaceutical and vaccine manufacturers. However, implementing disposables is only a partial step forward. Additional gains in speed, control, quality, flexibility, and overall cost can be obtained by implementing single-use operations within the FlexFactory platform from Xcellerex.
From its unique vantage point as both practitioner and platform designer, Xcellerex has challenged the foundations of bioproduction architecture. Rather than accept tradeoffs as unavoidable, Xcellerex sought new routes that eliminate barriers altogether. In addition to innovative hardware and software design, every FlexFactory system includes the know-how of experienced process development; regulatory; and process automation teams … all focused on helping clients get where they need to go — faster.
Xcellerex believes that biomanufacturing strategy is a cornerstone of its clients’ long-term prosperity. The company is dedicated to providing systems, tools, and resources to help bypass the tradeoffs that wait on the road to success.
You May Also Like






