Establishing Single-Use Assemblies on Filling EquipmentEstablishing Single-Use Assemblies on Filling Equipment
Biotest is a worldwide-operating company specializing in innovative hematology and immunology products with the holistic approach of a global pharmaceutical and biotherapeutics group. The company’s products are used to treat life-threatening diseases such as coagulation disorders (hemophilia), severe infections, and disorders of the immune system.
The most important starting material for Biotest’s pharmaceutical products is human blood plasma, which is processed into medicinal products at a production facility in Dreieich, Germany. The company has explored using single-use assemblies as part of an effective manufacturing strategy.
Single-use assemblies provide major advantages during clinical supply filling, when timing can be critical. A company can experience cost and time savings by eliminating cleaning and sterilization validations from project lead-in time. Some time-consuming cleaning and sterilization steps are not required because sterile single-use assemblies are used directly, allowing for significantly shorter process and set-up times.
Process-dedicated filling equipment is no longer required when a complete single-use filling system is used. Furthermore, the risk of cross-contamination is greatly reduced. That aspect is particularly important because Biotest’s filling line highlighted in this study is a heavily multifunctional line, used for the routine final filling of immune and hyperimmunoglobulin 2–50 mL preparations.
Preparation
To convert to single-use correctly, the new solution must respect a cleanroom’s specifications and enable the same process steps. Therefore, the single-use solution must include aseptic connections, a system of sterile filtration, filling needles, and connections to formulation bags.
To prepare for discussions with potential suppliers, the company conducted a detailed process review. Important themes include the connection to the filling line, the sterile filter (including single or redundant systems, preflushing requirements, and integrity testing), and the filling needle itself. In that early and very important phase, Biotest created a detailed “dummy model” of the single-use assembly. The proposed model was a complete solution that included the docking point to the product line, across the sterile filter to the disposable, aseptic filling needle, without additional interventions or connections to the sterile filter. The dummy model tested the product line, tubing length and diameter, position of the sterile filter and bags, and shapes and sizes of the filling needles.
Existing stainless steel equipment was a traditional filling line with a restricted access barrier system (RABS). The company performed sterile filling of final liquid drug products in a class A cleanroom within a class B background. Final formulated bulk was transferred from stainless steel mobile vessels through piped product lines, in which a single tube was guided to the multipurpose filling line and where the final product was sterile filtered.
For filling clinical trial material using single-use assemblies, final formulated bulk (delivered in a 100-L single-use bag) should be connected under class C laminar air flow to the sterile disposable tubing. Biotest carried out the actual connection of the single-use filling assembly to this product line in a class B cleanroom.
Choosing the Right Partner
Biotest considered three suppliers that the company had previously worked with over several years on projects that included work in sterile filtration and filter validation. All three suppliers are regularly audited by Biotest’s quality assurance department, which served as an added advantage for this supplier selection approach. For this special project, the company sought a supplier that could offer the most complete solution, taking into account the ambitious timing and success of the dummy model in the overall process. Ultimately, one supplier proposed a solution within a period of two weeks for implementation of the project.
Other priorities included the availability of data from extractables studies on the plastic components in the single-use assemblies, security of sourcing the single-use assembly, and cost considerations. Also considered to be important for the final filling step were assembly integrity and sterile filtration validation. After evaluating the results of the supplier negotiations and the intense scrutiny of the received solutions, Biotest selected EMD Millipore as a project partner.
As a division of Merck KGaA of Germany, EMD Millipore offers a broad spectrum of proven tools and technologies, together with performance solutions innovations, dedicated to helping customers succeed in the research, development, and production of biotechnology and pharmaceutical drug therapies. The company is a top-tier supplier to the life science industry that serves as a strategic partner for scientists, engineers, and researchers. EMD Millipore was the only supplier that offered a complete system, including an integrated sterile filter and a metal single-use filling needle.
From the Dummy Model to the First Drawing
The next step was to produce a drawing of the single-use assembly. Table 1 shows the timeline for completing this process. EMD Millipore generated the drawing, which defines product-contact components, materials of construction, dimensions, and certification level for the single-use assembly. The drawing outlined EMD Millipore’s change-control procedures and was subject to Biotest’s approval. Components to build the assembly were selected from a library of prequalified components.
Table 1

Table 1: 194;
Figure 1 shows the single-use assembly and its composition of several items. The main components were the sterile filter and the filling needle. Connections consisted of a main inlet to connect to the formulation bag, a spare inlet, and an integrity test connection for testing the sterile filter. A vent and flush bag enabled the integrity test of the sterile filter. A hold bag was added to ensure accuracy of dosing through the needle and prevent any discrepancy coming from the peristaltic pump.
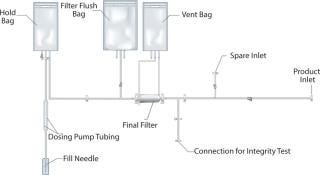
Figure 1:
EMD Millipore performed filter validatation of the sterilizing-grade final filter as a parallel phase of the project. The company included bacterial retention testing, product-specific filter integrity testing with both air and nitrogen, and chemical compatibility testing.
Bacterial Retention Testing: The filter’s ability to retain bioburden was qualified in combination with the product and its process parameters. Because product and/or processing conditions may be antagonistic to the test organism, bacterial retention testing was split into two parts: a viability test and the actual retention test.
Integrity Testing: The integrity testing of sterilizing-grade filters is a regulatory requirement (1,2). However, most drug products contain components that change surface interactions between the filter and the wetting fluid, which may alter integrity test results. So it is often useful to determine product-specific integrity value. Integrity test ratio determination (e.g., bubble-point ratio) is a proven method for providing minimum integrity values for a product-based wetting fluid.
Chemical Compatibility Testing: Numerous chemical interactions may occur between filter materials and constituents in a drug product. The effects of such interactions were characterized.
EMD Millipore produced the first prototypes on the basis of the first drawing. Several test runs dispensed water instead of product to check for suitability and functionality. In the test runs, the entire process was simulated and optimized — beginning with the transfer of the single-use assembly into the aseptic core through a vaporized hydrogen peroxide (VHP) lock through its disposal upon completion of the filling process. The focus of those test runs was the filling process with single-use equipment, in particular, compliance with the specified filling tolerances (Figure 2). Those test runs showed that two small changes would be required for completing the final drawing of the single-use assembly (Figure 1).
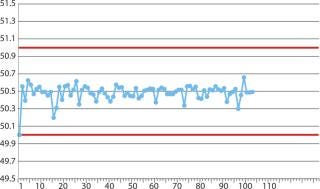
Figure 2:
During test runs, filling personnel were trained in proper handling of the new single-use filling assemblies. As with any implementation of an innovative solution such as single-use assemblies, training is a critical step to ensure user confidence and prevent mishandling. Moreover, such practical training for filling personnel early in development enables successful media fills later in the validation process.
EMD Millipore created a standard operating procedure (SOP) in parallel with the testing to describe the use of the single-use equipment for filling personnel and to serve as a basis for staff training. Implementation of single-use assemblies also requires specific attributes and data, especially in the final phase of a process such as final filling. Such validation must include extractables data to ensure the sterility and integrity of the single-use assembly.
Extractables: Extractables are compounds that can be extracted from elastomeric or plastic components under aggressive conditions in solvents of different physicochemical properties. Model solvents used for testing must be relevant to actual drug products with respect to ingredients and solvent vehicles. Commonly used model solvents include water, low- and high-pH water, and alcohol. The rationale for the selecting worst-case extraction conditions has to be justified, taking into consideration processing time, temperature, and sterilization conditions.
EMD Millipore performed a risk assessment to determine the availability of extractables data for the critical components based on process conditions provided by Biotest. For one project-specific component — a special silicone hose — no data were available, so it needed to be collected during the project.
Integrity of the Single-Use Assembly: For any part of the single-use filling assembly, it is imperative that a manufacturer be able to determine that the final assembly is integral before quality assurance (QA) release. EMD Millipore fill–finish assemblies are integrity tested using pressure decay before QA release. The automated test system is based on ASTM F2095, which requires a plate-restraint system for the container to allow high pressure to be applied to the entire assembly. In addition, the restraining plates minimize the fill volume of the assembly for greater test sensitivity.
Sterility of the Single-Use Assembly: The single-use assemblies are manufactured in an EMD Millipore facility, which adheres to good manufacturing practices (GMPs). The assemblies are exposed to a gamma irradiation level of 25–40 kGy. The sterilization process follows the ANSI/AAMI/ISO process (ANSI/AAMI ST32, ISO 11137) for establishing the dose map of the product, developing the dose run, and validating the irradiation run.
Media Fills and Successful Filling of Clinical Trial Material
Based on the details of the final approved drawing, the gamma-sterilized, triple-wrapped, single-use filling assemblies were delivered three months after the design was finalized. Before delivery, the inspection of incoming goods, the storage conditions, and the shelf life of the single-use assemblies were defined (usually, a shelf life of two years for functionality and sterility criteria is stated on the certificate of quality).
Operators validated single-use sterile filling equipment with three consecutive media fill runs. Just 10 months after the start of the project, the first batch of clinical trial material was manufactured using the single-use equipment (Photo 1). Thus, the task had been successfully implemented by the deadline.
Photo 1

Photo 1:
Table 2 lists the main milestones of this constructive and intensive collaboration. Such an achievement is possible only if the parties work together very closely as a team. This particularly applied to the cooperation of manufacturing operations at Biotest and technical experts at EMD Millipore.
Table 2

Table 2: 194;
Efficient Filling with Single-Use Assemblies
For a critical step such as final filling, implementing single-use can require specific validation such as generating extractables data, ensuring integrity and safety of an assembly, and training future users. However, as we described here, implementation of a single-use solution in the final filling step is feasible and has several benefits. It prevents cross-contamination without the need for cleaning, and it ensures a fast implementation without going through heavy capital investment and equipment qualification.
Author Details
Corresponding author Roland Gross, PhD, is senior director, pharmaceutical production (49-6103-801-0; [email protected]); Andreas Nink is assistant director, pharmaceutical production; and Frank Breitenbach is head of pharmaceutical manufacturing at Biotest AG, Landsteinerstr. 5, D-63303, Dreieich, Germany. Frans Mels is technical consultant, Jens-Uwe Hain is senior account manager, and Eric Youssef is European Plasma market manager at Merck Millipore, 39 Route Industrielle de la Hardt, 67120 Molsheim, France.
REFERENCES
1.) 2008.EudraLex: The Rules Governing Medicinal Products in the European Union Volume 4 — EU Guidelines to Good Manufacturing Practice Medicinal Products for Human and Veterinary Use: Annex 1 Manufacture of Sterile Medicinal Products, The European Commision, Brussels.
2.) 2004.Guidance for Industry: Sterile Drug Products Produced by Aseptic Processing — Current Good Manufacturing Practice, US Food and Drug Administation, Rockville, MD.
You May Also Like






