Seeking the Next Generation of Single-Use TechnologiesSeeking the Next Generation of Single-Use Technologies
April 1, 2014
Recent trends in biomanufacturing technology and the biopharmaceutical market are driving the increased adoption of single-use (SU) manufacturing systems. From the demand side, the biopharmaceutical industry’s focus on niche and rare diseases with relatively small patient populations is pushing for smaller, more flexible biomanufacturing capacities than have been needed in the past. The entry of many companies into biosimilars development also is leading to fragmentation and dispersion of manufacturing capacity. Changes on the supply side caused by technological advances have resulted in higher titers and enhanced specificity, again driving the industry toward smaller production systems. Furthermore, there has been increased outsourcing of manufacturing activities to contract manufacturing organizations (CMOs) whose multiproduct facilities have been the earliest adopters of SU technologies (1).
The benefits of SU technologies have been recounted in a multitude of articles and publications. Despite this publicity, opportunities remain for technical innovation, particularly with respect to downstream technologies.
Cost-Effective Solutions
Antibody products continue to dominate the biotech landscape, so protein A resin is a technology ripe for innovation (2). Despite the high level of adoption for protein A resin, its cost still accounts for most of the cost of antibody purification materials — >70% in some cases. That is particularly true for early phase and low-volume products, which are produced in only one to three batches at a time. The industry seems to have lost its appetite for developing non-protein A processes for antibodies. At the same time, the expiration of the protein A patent has led to a multitude of next-generation products produced by a number of companies: TOSOH, RepliGen, Kaneka, and JSR, among others. Such resins have shown comparable performance to established products in studies completed at my company. Those alternatives can also provide substantial cost advantages—more than 60% in some cases.
Despite the lower costs, higher flow rates, improved “cleanability,” and increased capacity of some new resins, no available option could be considered a plug-and-play chromatography solution for a single-use process. The cost of even the least expensive of those resins can present a major financial burden for smaller companies looking to generate preclinical and early phase clinical batches.
What’s still missing in downstream is a chromatography solution to streamline the process and reduce overall operational costs, not just resin costs. By incorporating the resin into a complete prepacked chromatography solution, total operating costs could be significantly reduced and productivity improved — bringing them more in line with other SU technology advancements. One example of this type of solution is the newly introduced Grace ProVance prepacked columns (Grace. com/ProVance).
Technical Hurdles
The clarification process — in which cellular debris are separated from the product stream — is a critical and often overlooked step in biomanufacturing. SU technologies are commonly used for bioreactors <500 L in volume. However, clarifying bioreactors >2,000 L in volume remains a technology gap for the industry. Increasing cell densities (such as >100 million cells/mL obtained during concentrated fed-batch operations) are compounding that struggle. Processing times required for disposable centrifuges can become quite lengthy if multiple parallel units are not used. In addition, centrifugation is often an ill-suited technology for extremely high cell densities because frequent bowl discharges are required, resulting in poor yields (3). The technology’s usefulness also may be challenged by lower-viability cultures, which often have poor cake-forming properties.
Increasing popularity of flocculation as a pretreatment before clarification has helped manufacturers address some of those issues (4,5,6). Although originally associated with hard-piped centrifugation systems, new products from Millipore are designed to pair flocculation with depth filtrations (7). That innovative solution substantially reduces filter-area requirements for bioreactors ≤2,000 L. Table 1 lists examples of the influence of this technology at Gallus. In addition, depth filtration is particularly attractive because (unlike centrifugation) it offers a scalable solution to clarification. Despite those wins, substantial challenges remain when clarification of bioreactors exceeding 2,000 L is necessary.
Table 1

Table 1: 194;
Size Matters
SU technologies have been readily available for production scales up to 1,000 L, which rarely yield more than 5 kg of product. Unfortunately, once bioreactors approach the 2,000-L range, challenges can arise, especially for high-titer processes, which can approach 10 kg. Table 2 lists column diameters required to process various batch sizes in less than three cycles at a loading of 50 g/Lresin. Columns up to 45 cm in internal diameter (ID) have only recently become available in a prepacked format. Such columns have shown excellent scalability with a very difficult separation (Figure 1). Although that advancement dramatically increases the applicability of prepacked columns for larger-scale biomanufacturing, a substantial gap still exists for high-gram batch sizes. In addition to the limited availability of large-scale columns, flow rates obtainable with pumps on the commonly available SU chromatography systems top out at those typically used for a 45 cm ID column.
Table 2

Table 2: 194;
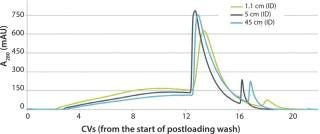
Figure 1:
Some basic process needs still have not been addressed with a disposable platform. Viral filtration is a unit operation used in nearly every process for biotherapeutics produced in mammalian cell culture (8). Although viral filters always have been disposable, the systems used to operate them have not. It is common to operate viral filters with peristaltic pumps, although they are not ideal for this pressure-driven operation because of their pulsing effect. In addition, the desire to operate some viral filters and pressures to 50 psi remains a challenge with SU pumps and most tubing.
Innovation in nonchromatographic separation techniques still has a long way to go. Membrane chromatography steadily increased in use for both clinical and commercial processes (9). Advantages of such technologies have been well documented and include decreased processing time from faster flow rates, reduced validation costs, and decreased water use. Membrane chromatography has had the highest level of adoption in flow-through applications in which trace impurities such as host-cell protein and DNA are removed. Membranes currently are not designed to deliver plate-based resolution; hence, their application is limited for separations that are more complex. Despite that, some examples exist, such as processes to purify Factor VIII, which use a membrane capture step. One pioneer in the field of so-called membrane chromatography is Natrix Separations, which manufactures a hydrogel absorber. Results have shown that this product can capture an antibody from clarified harvest at loading >80 g/L (10). In addition, Natrix is exploring the use of novel ligand chemistries that could further streamline downstream processing (NatrixSeparations.com).
Standardizing Documentation and Testing
Robust data packages from equipment manufacturers will need to become the norm, particularly if technologies are going to advance into late-phase and commercial processes. The time and money required for addressing questions around leachables and extractables can be substantial, especially for a disposable process.
The BioPhorum Operations Group (BPOG) is providing a forum for the biotechnology industry to converse and influence change on common issues. One subteam in this organization is specifically addressing concerns surrounding leachables and extractables for disposable parts. This state of affairs is not surprising given that the US Food and Drug Administration (FDA) provides no specific guidance on how to handle process-related extractables. Currently 13 companies and the American Society for Testing and Materials (ASTM) are working together to develop standard requirements for end users.
Moving forward
SU technologies are well established in bioprocessing and continue to grow. Biomanufacturers are increasingly recognizing the advantages of improved quality, safety from cross-contamination, flexibility, productivity, and time savings. Nevertheless, equipment suppliers should target a few specific areas for improvement and innovation.
Author Details
Paul Jorjorian is the senior manager of purification development at Gallus BioPharmaceuticals, LLC, 4766 LaGuardia Drive, St. Louis, MO, 63134-3117; [email protected].
REFERENCES
1.) 2013.Tenth Annual Report and Survey of Biopharmaceutical Manufacturing Capacity and Production, Bioplan Associates, Rockville.
2.) Gagnon, P 1996.Purification Tools for Monoclonal Antibodies, Validated Biosystems Inc, Tuscon:1-150.
3.) Roush, DJ, and L Yuefeng. 2008. Advances in Primary Recovery: Centrifugation and Membrane Technology. Biotech. Progress 24:488-495.
4.) Kang, YK. 2013. Development of a Novel and Efficient Cell Culture Flocculation Process Using a Stimulus Responsive Polymer to Streamline Antibody Purification Processes. Biotech. Bioeng. 110:2928-2937.
5.) Brodsky, Y. 2012. Caprylic Acid Precipitation Method for Impurity Reduction: An Alternative to Conventional Chromatography for Monoclonal Antibody Purification. Biotech. Bioeng. 109:2589-2598.
6.) Peram, T. 2010. Monoclonal Antibody Purification Using Cationic Polyelectrolytes: An Alternative to Column Chromatography. Biotech. Prog. 26:1322-1331.
7.) Singh, Nripen. 2013. Clarification of Recombinant Proteins from High Cell Density Mammalian Cell Culture Systems Using New Improved Depth Dilters. Biotech. Bioeng. 110:1964-1972.
8.) Eibl, D, T Peuker, and R Eibl. 2010.Single-Use Equipment in Biopharmaceutical Manufacture: A Brief Introduction Single- Use Technology in Biopharmaceutical Manufacture, John Wiley & Sons, New York.
9.) Bhut, BV, and SM Husson. 2009. Dramatic Performance Improvement of Weak Anion- Exchange Membranes for Chromatographic Bioseparations. J. Membrane Sci. 337:215-223.
10.) Jorjorian, P. 2013.Innovative Purification with Natrix™ Separation Technologies10th Annual Single-Use Applications for Biopharmaceutical Manufacturing, IBC Life Sciences, Durham.
You May Also Like






