Superior Scalability of Single-Use BioreactorsSuperior Scalability of Single-Use Bioreactors
During the past several years, single-use bioreactors have been gradually established in modern biopharmaceutical processes (1, 2). This adoption is directly linked to their unique ability to enhance flexibility and reduce investment and operational costs. Furthermore, production output can be increased, and time to market is shortened (3). Today a wide variety of single-use bioreactors exists for the cultivation of mammalian and insect cells (4), whereas only limited solutions are available for microbial cultures (5).

Figure 1: ambr250, UniVessel SU, and BIOSTAT STR family; working volume ranges from 250 mL to 2000 L
Typically, processes are established and optimized in stirred-tank benchtop bioreactor systems. One challenge during the development of a robust cell culture process is the straightforward scale-up to final production scale. This is especially critical when using less-characterized bioreactor designs that deviate from the well-known and understood classical stirred-tank principle.
Scale-up is an important and potentially time-consuming step in the development of industrial processes. It involves much more than just doing the same at a larger volume. It requires the generation of solid process understanding at different scales to ensure consistent quality and titer throughout scale-up from early clinical trials to final production scale (6). Today, many companies use chemometric tools such as design of experiments and multivariate data analysis to establish critical process parameter ranges that define the design space of a robust production process. Especially during late-phase development of a commercial process, the availability of a properly representative scale-down model of full production scale is essential to allow efficient process development (7).
Detailed understanding of bioreactor characteristics at different scales significantly facilitates the development and scale-up of robust production processes (6). Typical parameters of concern are oxygen transfer, mixing, and heat-transfer characteristics as well as the generated shear forces.
During the past 30 years, stainless-steel stirred-tank bioreactors have evolved as the gold standard, especially as a result of their straightforward scale-up. Multiple times, their well-understood design principles have proven successful in development and scale-up to safe and robust commercial processes. Furthermore, they enable users to implement their existing knowledge — especially with platform processes — into production processes of new drugs and to set-up experiments in a way that can shorten development timelines.
However, many commercially available single-use bioreactors differ from this gold standard. Vessel design, stirrer design, and gassing strategy especially may differ from the classical stirred-tank design principles and do not necessarily offer consistency and geometrical similarity across scales (8). So the scale-up exercise might be complicated, and additional risk might be added to the process transfer.
To offer a solution for that, Sartorius Stedim Biotech has developed a range of single-use bioreactors from 250 mL to 2,000 L working volume. Its designs are entirely based on proven stirred-tank bioreactor principles. This ensures straightforward scale-up to 2,000 L scale and beyond to facilitate process transfers to existing legacy production facilities.
Here we detail the different design aspects of those bioreactors and their impact on critical process engineering parameters such as power input per volume, mixing time, and volumetric mass transfer (9). We compare the characteristics of the large scale single-use bioreactor family BIOSTAT STR to small-scale single-use bioreactor vessels such as the ambr250 (250 mL) and the single-use UniVessel SU 2L, which are typically used during process development, optimization, and characterization.
Design Principles and Suitable Scale-Up Criteria of Stirred-Tank Bioreactors
Geometrical similarity of vessel design (amongst others defined by the height-to-diameter ratio and the impeller-to-vessel-diameter ratio) is commonly considered important for simple and straightforward scale-up of a process (10). This is especially critical as design changes across scales might influence mixing behavior, oxygen transfer, bubble dispersion, and various other key parameters. On the other hand, a homogenous culture environment across scales — where important cultivation parameters such as pH, oxygen partial pressure, temperature, and nutrient supply are well controlled — is a key prerequisite to establish a robust and safe production process. To characterize bioreactor performance across scales and govern scale-up, an appropriate criterion should be defined and kept constant during scale-up. In general, the power input per volume is used as scale-up criterion (10). Also, the tip speed or other shear-related parameters are often used, especially when using shear-sensitive cells (11) or when growing cells on microcarriers.
Based on the very well-characterized, reusable, stirred-tank bioreactors, it is possible to assign relevant design criteria to single-use bioreactors for animal and microbial cells. One such criterion is the height-to-vessel-diameter ratio (H/D or aspect ratio), which should be kept within a range of 1:1 to 3:1 for stirred-tank reactors (10). A low value for an H/D ratio results in an increased ratio of headspace surface to filling volume, which enables an
improved gas exchange at the gas– liquid interface. On the other hand, a larger aspect ratio offers advantages in case of direct sparging due to the longer residence time of gas bubbles in the liquid and hence a higher oxygen-transfer rate (12). For animal cell cultivations, often a ratio of 2:1 is recommended (13).
Another parameter to consider is the ratio of impeller diameter to vessel diameter. This parameter should typically be between 0.33 and 0.5 for animal cells (14) and influences mixing efficiency and the generated shear forces. Three-blade-segment impellers or marine-type impellers are commonly used for animal cell cultures (15). They efficiently transform the transferred energy into hydrodynamic power and generate large circulation loops because of their axial flow patterns (16). Therefore, they are often preferred over Rushton-type disk impellers to achieve effective homogenization. Also, because of the lower dissipative energy transferred, this impeller type is more adequate for shear-sensitive cell lines. In comparison, disk impellers generate a radial flow, leading to higher power input per volume at a given stirrer speed and enhanced gas-bubble dispersion (11). It is common to install multiple impellers in bioreactors with an H/D ratio above 1:1.4 to ensure efficient mixing throughout the entire cultivation chamber.
To achieve suitable power input, the distance between the impellers is important. It is recommended to use a distance that is 1.2–1.5 times greater than the impeller diameter to guarantee that the impellers act independently of each other. Historically cell culture processes often used two three-blade segment impellers, but today many companies are using a combination of a Rushton-type impeller and a three-blade segment impeller. The disk impeller (installed right above the sparger) ensures good dispersion of the gas bubbles. A three-blade segment impeller serves as a superior axial mixer and ensures homogeneity in the entire vessel, thus supporting high–cell-density processes (17, 18). That approach has been facilitated by modern, more robust recombinant cell lines to grow at higher shear rates, which have been selected for commercial manufacturing at large scale (19).
Another determining factor for a successful cell culture process is the amount of gas transfer. In conventional bioreactor designs, a sparger is installed directly below the lower impeller, which ensures proper gas-bubble dispersion (14). Spargers with small holes generate small bubbles and improve oxygen transfer because of their high gas–liquid interface areas. Reusable bioreactors use sintered stainless steel microspargers. But this design has the disadvantage of nondefined pores, leading to coalescence of bubbles. Therefore, Sartorius Stedim Biotech
has developed a special microsparger design with 150 μm holes that provides a uniform bubble swarm of small bubbles for effective gas transfer. Spargers with large holes have a relatively low oxygen transfer but offer improved performance for CO2 stripping because bigger bubbles typically rise to the gas–liquid interface and carry excessive CO2 from the cell suspension to the headspace.
At small-scale, CO2 stripping is less of a challenge. It is more critical at larger volumes because of the higher hydrostatic pressure and thus improved solubility of CO2. Together with excessive foaming, that limits the efficiency of conventional 10 to 20 μm microspargers for large bioreactor volumes.
Sartorius Stedim Biotech Single-Use Bioreactors Emulate Classical Stirred-Tank Design
Most large-scale, single-use bioreactors do not rely on established design criteria of reusable bioreactors, which can add risk to scaling-up processes. To overcome this, Sartorius Stedim Biotech offers a range of stirred single-use bioreactors (Figure 1) based on classical, well-proven design principles. Different scales exist, allowing to work from 250 mL to 2,000 L culture volumes.
For efficient, cost-conscious process development, highly automated, single-use multiparallel bioreactors are available at 250 mL scale (ambr250) (20). This high-throughput process development bioreactor system allows fast and effective establishment of optimal process conditions early in process development. With the classical stirred-tank design, straightforward scale-up is possible either directly for production of material for toxicological studies or through step-wise scale-up through 2 L scale using the UniVessel SU technology and 50 L scale using the BIOSTAT STR. With the UniVessel SU 2 L model, conventional glass vessels can be replaced easily even with already existing bioreactor controllers in development laboratories. The UniVessel SU design is similar to its glass counterpart and the large-scale single-use BIOSTAT STR design, thereby enabling straightforward scale-up all the way from development to commercial production and offering a single-use scale-down model for process characterization.
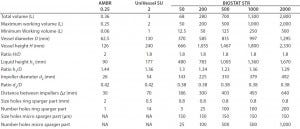
Table 1: Summary of geometrical dimensions of the ambr250, UniVessel SU, and BIOSTAT STR family
The BIOSTAT STR has a cylindrical shape with an H/D ratio of 2:1 and a semi-torispherical bottom and top. The impeller-to-bag-diameter-ratio is 0.38 with a distance between both impellers of 1.3× the impeller diameter. So the vessel design fits perfectly to the gold standard derived from reusable bioreactors for mammalian cell culture. Table 1 provides a detailed description of the geometrical dimensions of those closely linked single-use bioreactor families.
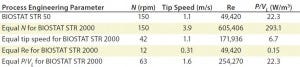
Table 2: Comparison of process engineering parameters suitable for scale-up from the BIOSTAT STR 50 to the BIOSTAT STR 2000; for scale-up, a CHO process performed at 50 L scale was assumed, which was performed at 150 rpm equivalent to a tip speed of 1.1 m/s, a commonly used tip speed for cell culture applications
The impellers are installed on a rigid, central shaft. For agitation, 2 × 3-blade-segment impellers are available. For the BIOSTAT STR, a combination of a six-blade-disk (bottom) and three-blade-segment (top) impeller can be installed as an alternative.

Figure 2: Combisparger for the BIOSTAT STR
2000L system
The study presented here focuses on process engineering characterization of a configuration based on 2 × 3-blade-segment impellers for different single-use bioreactor volumes. Gas transfer has been characterized for a microsparger (hole diameter = 150 μm) or a ringsparger (hole diameter = 0.5 mm for the Univessel SU or 0.8 mm for the BIOSTAT STR) positioned below the lower impeller. The BIOSTAT STR is available with a combisparger (Figure 2) — consisting of both a microsparger (0.15 mm holes) and a ringsparger (0.8 mm holes). The microsparger supports high oxygen transfer, and the ringsparger enhances CO2 stripping. All single-use bioreactors from 250 mL scale to 2,000 L are equipped with precalibrated, single-use optochemical probes for pH and pO2 measurement. Alternatively, conventional probes can be introduced if desired for scales ≥2 L. All single-use bioreactors are available with standard digital control units.
Process Engineering Characterization
We performed in-house process engineering characterization of the UniVessel SU and BIOSTAT STR bioreactors. For the ambr250 system, we used data published by Bareither et al. (20). The process engineering characterization of the UniVessel SU and BIOSTAT STR family was performed at parameters typical for mammalian cell culture (tip speeds between 0.6–1.8 m/s). For the characterization of the ambr250 Bareither et al. used tip speeds ranging from 0.27 m/s to 1.02 m/s, which resulted in corresponding stirrer speeds from 200 to 800 rpm (20).
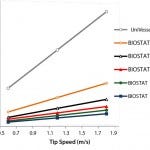
Figure 3: Stirrer speed as a function of the tip speed for the UniVessel SU and BIOSTAT STR
reactors.
Figure 3 graphs stirrer speed as a function of tip speed for the UniVessel SU and BIOSTAT STR systems. The increasing impeller diameters requires lower stirrer speeds at increasing scale to maintain the same tip speed.
During in-house trials for the BIOSTAT STR and UniVessel SU bioreactors, the Newton number (Ne) was determined to characterize the different impeller types and configurations and to quantify the power input per volume. The Newton number was determined by torque measurements (8). For 2 × 3-blade segment impellers, we calculated a Ne of ~1.3. The fact that the Reynolds number (Re) is above 10,000 at the chosen tip-speed range implies that turbulent flow conditions are present, thus the Ne value is constant.
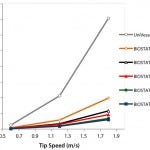
Figure 4: Power input per volume (P/VL) for the UniVessel® SU and BIOSTAT® STR family
The power input per volume (P/VL) is an important process engineering parameter and can be calculated based on the experimentally determined Newton number. Figure 4 shows the power input per volume for the BIOSTAT STR and the UniVessel SU bioreactors. For the ambr system, Bareither et al. (20) reports a power input of 10–445 W/m³ for the tip speed. To maintain a constant P/VL value during scale-up, the tip speed will need to increase with increasing scale. That clearly demonstrates that users have to choose their scale-up criterion because it is not possible to keep both the tip speed and the power input per volume constant when increasing the bioreactor scale.
We determined mixing times for the BIOSTAT STR and UniVessel SU bioreactors using the decolorization method (14) to characterize the mixing capabilities of the single-use bioreactor systems. It is obvious that the mixing time decreases with increasing tip speed because of the higher power input per volume. In addition, we obtained higher mixing times with increasing scales.
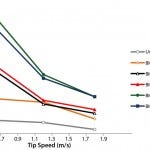
Figure 5: Mixing times for the different scales of the UniVessel SU and BIOSTAT STR family as a function of the tip speed for 2 x 3-blade-segment impeller configuration
Figure 5 shows the mixing efficiency for the different single-use bioreactor scales. At 2 L scale, a mixing time of 7 s at a tip speed of 0.6 m/s and 2 s at a tip speed of 1.8 m/s can be achieved. At 50 L scale, mixing times increase to 19 s at tip speed of 0.6 m/s and 8 s at a tip speed of 1.8 m/s. For 2,000 L scale, mixing times of 20 s can be obtained. Overall, it is possible to ensure mixing times <30 s for all scales, from 2 L to 2,000 L, thus demonstrating the superior performance of the UniVessel SU and BIOSTAT STR family for mammalian cell culture (9).
For the quantification of the oxygen-transfer rate, we determined the volumetric mass transfer coefficient (kLa) using the gassing out method (21). For most common cell cultures, kLa values of 5–10 h–1 are required. Figure 6 shows the kLa characteristics for different tip speeds and scales of the BIOSTAT STR and UniVessel SU bioreactors. Both ring and microsparger elements were used for the BIOSTAT STR system and a ringsparger for the UniVessel SU and ambr studies.
The kLa-values increase with increasing tip speed at any given scale and configuration. As expected, the kLa values increase also with the scale. That can be explained by the increased liquid height and the longer residence time of the gas bubbles in the liquid — resulting in a more efficient oxygen
transfer (22). The ambr system had kLa values of 8.5 h–1 at a tip speed of 1.02 m/s (20). For the other bioreactor families kLa values >10 h–1 can be easily achieved at all scales for both microsparger and ringsparger. Hence, the ambr, UniVessel SU, and BIOSTAT STR bioreactors meet the oxygen-transfer requirements of mammalian cell cultures. With a microsparger, kLa values up to 40 h-1 can be reached at 2,000 L scale, thereby demonstrating the superior performance of this bioreactor type and making this bioreactor type the ideal choice for high cell-density processes.
Superior Single-Use Bioreactor Scalability Due to Classical Stirred Tank Principles
Their excellent performance characteristics make the single-use ambr250, UniVessel SU, and BIOSTAT STR bioreactors ideal for mammalian cell culture — even for very demanding, high cell-density or microcarrier-based processes. Low-shear agitation with three-blade segment impellers provides homogeneous mixing. With a nonparticle-shedding microsparger, operators can reach oxygen transfer rates of up to 40 h–1 at 2,000 L scale. Like other single-use bioreactors, such systems make cell culture operations more flexible, cost-effective, and less time-consuming. Their classical stirred-tank design allows relying on well-known and established scale-up criteria such as power input per volume or tip speed. In addition, their geometrical similarities across all scales facilitate successful scale up and scale down as well as process transfer, thus derisking scale-up and process transfers significantly. These single-use stirred tank bioreactor solutions enable seamless scale-up from 250 mL to 2,000 L. This makes them ideal solutions for any modern biopharmaceutical development and production facility for monoclonal antibodies, recombinant proteins, and vaccines.
References
1 Eibl R, Werner S, Eibl D. Bag Bioreactor based on Wave-Induced Motion: Characteristics and Applications. Adv. Biochem Engin. Biotechnol. 115, 2009: 55–87.
2 Brecht R. Disposable Bioreactors: Maturation into Pharmaceutical Glycoprotein Manufacturing, Applications. Adv. Biochem. Engin. Biotechnol. 115, 2009: 1-31.
3 Eibl D, Peuker T, Eibl R. Single-Use Equipment in Biopharmaceutical Manufacture: A Brief Introduction. Single-Use Technology in Biopharmaceutical Manufacture. Eibl R, Eibl D, Eds. Wiley: Hoboken, NJ, 2010.
4 Eibl R, et al. Disposable Bioreactors: The Current State-of-the-Art and Recommended Applications in Biotechnology. Appl. Microbiol. Biotechnol. 86 2010: 41–49.
5 Dreher T, et al. Microbial High Cell Density Fermentations in a Stirred Single-Use Bioreactor. Adv. Biochem. Eng. Biotechnol. 138, 2013: 127–147.
6 Junker BH. Scale-Up Methodologies for Escherichia coli and Yeast Fermentation Processes. J. Bio. Bioeng. 97(6) 2004: 347–364.
7 Mire-Sluis A, et al. New Paradigms for Process Validations. BioProcess Int. 11(9) 2013: 28–39.
8 Löffelholz C, et al. Bioengineering Parameters for Single-Use Bioreactors: Overview and Evaluation of Suitable Methods. Chemie Ingenieur Technik 85(1–2) 2013: 40–56.
9 Lara A R, et al. Living with Heterogenities in Bioreactors. Mol. Biotech. 34(3) 2006: 355–381.
10 Platas Barradas O, et al. Evaluation of Criteria for Bioreactor Comparison and Operation Standardization for Mammalian Cell Culture. Eng. Life Sci. 12, 2012: 518–528.
11 Storhas W. Bioreaktoren und Periphere Einrichtungen. Vieweg & Sohn Verlagsgesellschaft: Braunschweig/Wiesbaden, Germany, 1994.
12 Catapano G, et al. Bioreactor Design and Scale-Up. Cell Culture and Tissue Reaktion Engineering: Principles and Practice. Eibl R, et al., Eds. Springer-Verlag Berlin Heidelberg: Berlin, Germany, 2009.
13 Stanbury PF, Whitaker A, Hall J. Principles of Fermentation Technology. Pergamon Press Oxford: New York, NY 1995.
14 Marks D. Equipment Design Considerations for Large-Scale Cell Culture. Cytotechnol. 42, 2003: 21–33.
15 Fenge C, et al. Agitation, Aeration, and Perfusion Modules for Cell Culture Bioreactors. Cytotechnol. 11, 1993: 233–244.
16 Zlokarnik M. Rührtechnik Theorie und Praxis. Springer-Verlag Berlin Heidelberg, Berlin, Germany, 1999.
17 Mol O. Meeting Increased Media Throughput and Enhanced Process-Control with Single-Use Solution for Meeting Challenges in Extremely High Cell Densities. Single-Use Forum, Istanbul, Turkey, 5 July 2013.
18 Zijlstra G, et al. High Cell Density XD Cultivation of CHO Cells in the BIOSTAT CultiBag STR 50 L Single-Use Bioreactor with Novel Microsparger and Single-Use Exhaust Cooler; http://microsite.sartorius.com/ fileadmin/Image_Archive/microsite/ BIOSTAT®_cultibag_str/pdf/11-06-21/ DSM_ESACT_Sartorius.pdf.
19 Ozturk S. Cell Culture Technology: An Overview, Cell Culture Technology for Pharmaceutical and Cell-Based Therapies, 2006.
20 Bareither R, et al. Automated Disposable Small-Scale Reactor for High Throughput Bioprocess Development: A Proof of Concept Study. Biotechnol. Bioeng. 110(12) 2013: 3126–3138.
21 Wise WS. The Measurement of the Aeration of Culture Media. J. Gen. Microbio. 167–177, 1951, DOI: 10.1099/00221287
22 Ochoa FG, Gomez E. Bioreactor Scale Up and Oxygen Transfer Rate in Microbial Processes: An Overview, Biotech. Adv. 27, 153– 176, 2009, DOI: 10.1016/j.biotechadv.2008. 10.006.
Corresponding author Davy De Wilde is director of marketing, fermentation technologies; Thomas Dreher is scientist, R&D upstream technology; Christian Zahnow is scientist, R&D upstream technology; Ute Husemann is R&D manager, upstream technology; Gerhard Greller is R&D director, upstream technology; Thorsten Adams is product manager, fermentation technologies; and Christel Fenge is vice president of marketing, fermentation technologies at Sartorius Stedim Biotech.
You May Also Like





