The Green Imperative: Part Two — Engineering for Sustainability in Single-Use TechnologiesThe Green Imperative: Part Two — Engineering for Sustainability in Single-Use Technologies
 In BPI’s June 2020 issue, the first installment of this series introduces the study and implementation of single-use (SU) technology to provide a more sustainable manufacturing environment (1). We presented evidence showing that the economic and social benefits of SU systems currently outweigh the residual environmental risks. Not only is SU technology often a better environmental choice than traditional biomanufacturing options, it also is sometimes the only choice for rapid process design and facility start-up. In situations such as the current pandemic, SU systems are instrumental to developing new drugs and vaccines quickly and safely.
In BPI’s June 2020 issue, the first installment of this series introduces the study and implementation of single-use (SU) technology to provide a more sustainable manufacturing environment (1). We presented evidence showing that the economic and social benefits of SU systems currently outweigh the residual environmental risks. Not only is SU technology often a better environmental choice than traditional biomanufacturing options, it also is sometimes the only choice for rapid process design and facility start-up. In situations such as the current pandemic, SU systems are instrumental to developing new drugs and vaccines quickly and safely.
Below we outline current thinking on how to design materials, systems, and processes to support the “rethink, reengineer, reduce, reuse, and recycle” paradigm of the circular-economy concept for plastic and packaging. Through engineering efforts to improve sustainability, SU technology will become an even better manufacturing technology option in the future. In an upcoming issue, the final installment of this series will describe current and future “end-of-life” handling methods and reprocessing technologies for SU systems and components.
The New Plastics Economy
In January 2016, a report on “the new plastics economy” was published by the World Economic Forum and the Ellen MacArthur Foundation with analytical support from McKinsey and Company (2). The report invited the plastic-packaging industry to transition from a linear- to a circular-economy approach that will recover value from plastic materials and help protect the environment. The new plastics economy initiative (Figure 1) encourages manufacturers and marketers to foster innovation in the plastic industry by adding “rethink” and “reengineer” stages to the traditional “reduce–reuse–recycle” waste reduction strategy. “Rethink” and “reengineer” actions should be applied at all stages of the lifecycle for these products: from conceptual design to the end of life.
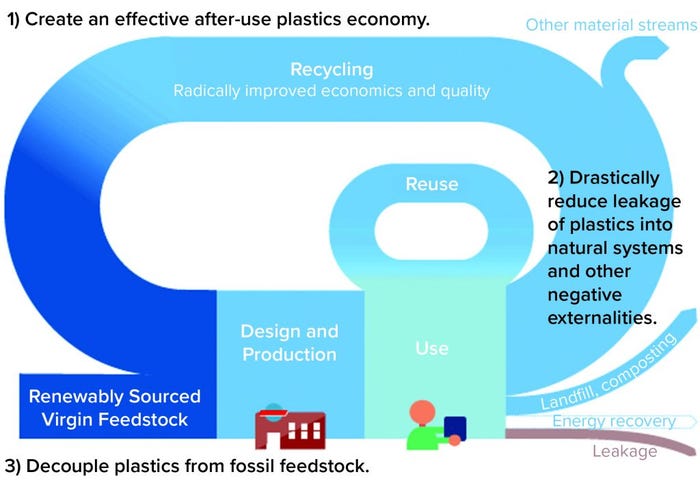
Figure 1: New plastic economy circularity guidelines (1, 2)
The report encourages manufacturers, industries, and national organizations to set and document ambitious goals in a “plastic pact” (3). As public concerns about environmental issues such as global warming and waste have increased in recent years, governments and policy makers have begun to draft laws and guidelines for pushing the plastic industry to adopt strategies outlined in the report.
Plastic and Healthcare
Even though SU applications in healthcare are rarely criticized — and despite the fact that plastic packaging used in healthcare products represents under 2% of total plastics produced each year — circularity guidelines for packaging do not exclude pharmaceutical packaging from their scope (4). As the World Health Organization has stated, “Of the total amount of waste generated by healthcare activities, about 85% is general, nonhazardous waste” (5). A significant amount of plastic used in healthcare can be reclaimed through standard recycling means.
In the business-to-consumer market, the pharmaceutical industry is addressing the use of plastic responsibly and creatively by introducing circular-design principles for final packaging. For example, Boehringer Ingelheim’s reusable inhaler and Huhtamaki’s recyclable blister packaging for tablets both received awards during Pharmapack Europe 2020.
In the biopharmaceutical industry, SU facilities increasingly are recognized to be more ecofriendly than traditional facilities. However, the rising use of SU technology is expanding the quantity of plastic waste generated in biomanufacturing. Managing that material according to circular-economy principles is becoming more important as SU consumption continues to grow. Below, we present concepts and practices that can help the SU and biopharmaceutical industries move toward that circular economy. Product design, selection of raw materials with an eye toward recyclability, reduction of materials, reuse opportunities, improved SU production practices, and improvements in process design and biomanufacturing use of SU are addressed. Postuse processing of SU waste and reprocessing opportunities will be covered in our third and final installment in this “Green Imperative” series.
Circularity in Design and Manufacturing
It is important to include entire SU systems when considering how to move toward a circular-economy paradigm. The main objective of product design is to guarantee that a product functions as intended at every step of its lifecycle. SU technologies need to meet a number of requirements (6): They must withstand gamma irradiation, maintain integrity during transportation and storage, serve their function as bioprocessing system components, and be manageable as process waste. Trade-offs between bioprocess performance and recyclability must be identified and assessed. Material engineers’ goal is to meet both sets of requirements, but achieving that goal is a challenge.
Biopharmaceutical industry users expect attributes such as mechanical robustness and chemical resistance to meet process needs across a wide range of conditions. Material choices are influenced by the need to operate from –80 to +60 °C. Product-contact materials must be designed to minimize extractables, and particular attention has been given to the material chemistry of bag films for SU containers (7). Minimizing extractables is not necessarily compatible with optimizing other performance attributes or with the goal of recyclability. Some material selections are tied directly to performance of SU systems in mixing, draining, filtration, and other process applications. And customers are interested in long shelf lives of SU components, which puts further demands on material engineers.
Packaging is part of a SU product. It is intended to protect the processing components from mechanical stress and contamination during transportation and handling from the end of SU manufacturing to the point of use. SU components typically are shipped in cardboard boxes and often are packaged within two heat-sealed plastic pouches. One pouch is removed as the SU is transferred through an airlock into a biomanufacturing clean room, and the other is removed at the point (and time) of use.
Note that packaging materials can be collected without biocontamination and are recyclable in standard collection and reprocessing streams. Additional packaging items such as bubble wraps, foam sheets, tape, and cable ties used to organize and protect subcomponents of complicated assemblies such as bioreactors and mixers also may be recyclable. For some applications, packaging must be resistant to cleaning agents such as isopropanol, aqueous disinfectants, and sporicides. Resistance to vaporized hydrogen peroxide (VHP) is important for SU components used in isolators. Cleaning and disinfection requirements could force designers to select less-commonly recycled materials such as multilayer film bags. The ratio of plastic weight involved in packaging to that of the product is usually ~10%.
Evaluating Consumption |
|---|
Plastic-component manufacturing plays a large role in plastic reduction. Technologies such as injection molding or additive manufacturing are better material-saving options than machining, which requires removal of material that becomes waste. Controlled and optimized plastics manufacturing also decreases plastic waste. Scrapless manufacturing processes (e.g., hot-runner injection molding) are preferable to those that generate waste (e.g., cold-runner injection molding). Designers need to solve conflicting requirements and make trade-offs. The struggle to optimize protective films for food packaging is a good example. By reducing the amount of plastic — the primary goal of a circular economy — food-packaging films have evolved from relatively thick single layers to thinner multilayer films that improve the shelf life of foods. In exchange for lowered material consumption and the important reduction of food spoilage and waste, the food industry increasingly is using films that are not recyclable in standard collection and reprocessing streams. Packaging material use can be optimized by increasing the number of products contained within the same package. Bulk packaging serves not only to reduce the amount of packaging per product, but also to decrease a product’s footprint during shipping and storage. Design of standard SU modules would enable packaging optimization. When new components are integrated into a manifold, the handling ability of integrators and end users should be considered, along with inclusion of multiple materials and their impact on postuse processing. The number of connection points that could be damaged during shipping and handling should be limited and components incorporated that address multiple packaging scenarios. |
“Reduce” and “Recycle” Circular Goals: From material-design and management perspectives, reducing the amount of material while meeting specifications without compromising product functionality is part of design best practices. That applies to both SU processing components and their packaging. Evaluating plastic consumption through a product lifecycle highlights opportunities for reducing plastic waste. Some examples of how to apply that evaluation process (“rethinking” and “reengineering”) are listed in the “Evaluating Consumption” box.
Recycling has a two-part impact on material selection: Products can be designed with recyclable materials and/or integrate recycled materials. Briefly described herein to help understand their impact on materials selection, these methods will be detailed further in the conclusion of this series.
The new plastic economy strategy encourages use of recycled materials and (when that is not possible) manufacture of plastics from renewable feedstocks such as sugar cane. An example of plastic made from renewable feedstock is plant-based polyethylene terephthalate (PET) used in some beverage containers. Most SU products (and their plastic packaging) used today are manufactured from virgin material (first-use, not recycled) that comes from fossil-fuel feedstock. Opportunities to integrate recycled materials and/or plastics made from alternative feedstocks in SU products should be explored.
Material Selection: The generic term plastic covers many materials designed to meet very different needs for thousands of customized user-defined applications. Plastics are synthetic materials made from a wide range of organic polymers such as polyethylene (PE), polypropylene (PP), polyvinyl chloride (PVC), polycarbonate (PC), and many others. Those are mixed with additives, chemical compounds that protect polymers and improve material performance. Oxidation and UV light resistance, fire-retardant properties, and physical properties such as puncture resistance all can be adjusted with suitable additives. A complete chapter of the MacArthur Foundation report is dedicated to plastic materials safety in all product lifecycle phases, including removal of all substances of concern (2). Removing additives that are unsuitable for use in the biopharmaceutical industry has been a focus of much effort in development of industry-specific materials such as bag films. Bag-film engineering has produced a number of plastics and combinations thereof designed to meet the specific requirements of bioprocessing.
When designing SU products, manufacturers often distinguish between product-contact and non–product-contact materials (e.g., external components layers, packaging, and other external parts). Designers must consider bioprocess risks to select the best materials that will contact bioprocess fluids. For example, priority must be given to materials with low extractables and an absence of harmful additives.
Materials used in the bioprocess industry are selected carefully to comply with pharmacopoeias and regulations such as REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) in Europe. The latter prevents plastic-containing impurities such as bisphenol A (BPA), phthalates, melamine, and so on from being used in the manufacturing of drug products. By design, the REACH regulation aligns with plastic material safety as stated in the circular economy guidelines (2).
In addition to patient safety, SU designers must consider bioprocess performance. For example, after cell growth issues were reported by users of SU bags, a new generation of film was developed (8). An antioxidant is required to protect polymers during film manufacturing, gamma irradiation, and storage. A legacy antioxidant was identified as the cause of the cell growth difficulties. In response, alternative antioxidants were evaluated and their concentration optimized to achieve the necessary film properties for cell culture use. Next-generation films can be used without releasing harmful byproducts during their lifecycle, and they can be produced with batch-to-batch consistency (9). Change-control procedures are in place with all stakeholders along the supply chain to ensure that impurity profiles remain unchanged and that harmful ingredients are absent.
Plastic Recycling Methods: The new plastic economy defines two recycling methodologies: mechanical and chemical recycling (Figure 2). Neither is “better” than the other; they complement each other, and each is used when and where it makes the most sense to do so.
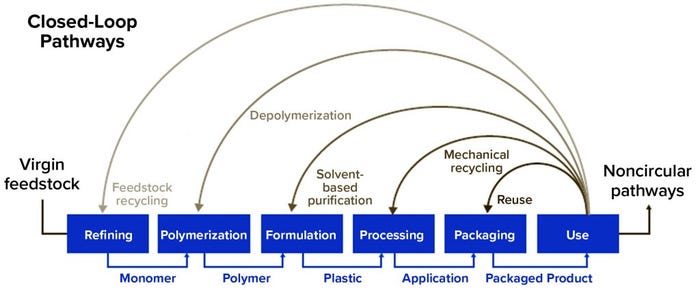
Figure 2: Overview of different loops for plastics in a circular economy (11) by consultant Mats Linder (MLSH Consulting AB)
Mechanical recycling of plastic is a multistep approach. For best results, the plastic must be as pure as possible. It is collected, sorted, shredded, and cleaned — then melted, extruded, and pelletized to be used again as a raw material. Not all plastics can be recycled this way. The two main categories of polymers are thermoplastics and thermosets. Thermoplastics (e.g., polyethylene) are polymers that can be melted when heated and hardened when cooled. Those characteristics are reversible and repeatable, allowing for mechanical recycling to convert used plastic into new plastic products. By contrast, thermosets (e.g. silicone) are polymers that undergo a chemical change when heated. After they are heated and formed, thermosets cannot be remelted and reformed.
Primary mechanical recycling of plastics converts thermoplastic polymers into products with equivalent properties. Such closed-loop processes can be applied only for plastics that have not been used at all or that have been decontaminated thoroughly before recycling. Secondary mechanical recycling generally yields products of lower mechanical properties than those of the starting materials. Mixed thermoplastic wastes and multilayer films used in packaging sometimes can be mechanically recycled and reused in lower-value applications such as building and construction.
Chemical recycling breaks down polymers into individual monomers or other hydrocarbon products that serve as building blocks or feedstock to produce new polymers. This includes solvent-based purification, depolymerization, and feedstock recycling. The latter describes thermal processes that convert polymers into simpler molecules by either pyrolysis or gasification. Theoretically, chemical recycling can process mixed materials to generate virgin-quality polymers.
Organic recycling applies aerobic (composting) or anaerobic (biomethanization) treatment under controlled conditions using microorganisms to biodegrade — not to be confused with biosource (Table 1) — plastic waste and produce stabilized organic residues or methane. As mentioned above, this emerging recycling method fits into a circular economy only through the idea of closing the cycle if biological feedstock is used. However, because of unproven robustness and concerns about sterilization and shelf life, using biodegradable polymers for SU product-contact components raises concerns about validation and risk. Implementation of biodegradable materials has low priority for the SU and biopharmaceutical industries.
Designing for recycling has been a long-time goal of the automobile industry (11). Efforts there have resulted in vehicles that are easy to dismantle, thus facilitating material separation. The new plastics economy is pushing designers to integrate design-for-recycling concepts into the biopharmaceutical industry, especially for SU products and packaging.
SU products first must be fit for purpose, which involves trade-offs between competing design requirements (recyclability being just one of those). Materials of construction are key to the recyclability of a product. Plastics are classified into seven categories according to resin identification codes (RIC), a system described in ASTM D7611 (12). Based on their RICs, products can be recycled properly while preserving their value.
ASTM D7611 provides codes for six commonly used resin types, with a seventh category created for all other types. The categories are polyethylene terephthalate (PETE); high-density polyethylene (HDPE); polyvinyl chloride (V); low-density polyethylene (LDPE); polypropylene (PP); polystyrene (PS); and others, including materials made with more than one resin from categories 1–6. Most plastics used in SU systems for biomanufacturing are polyolefins such as LDPE (4 in Figure 3) or PP (5 in Figure 3). Bag films and filters are significant contributors to plastic weight.

Figure 3: The ASTM International Resin Identification Coding (RIC) system facilitates recycling of plastics (12).
Although thermoplastic items are recyclable in theory, few of them are recycled in practice. Most have not been designed for compatibility with the current recycling infrastructure. The products that are most easily recycled are those produced from a single-grade thermoplastic. That is relatively straightforward for single-component items, but most users of industrial products have more complex geometric and functional requirements that cannot be fulfilled by just one material. Complicated SU products such as bioreactors and mixers have multipiece and multimaterial constructions. For such applications, design approaches for simplified disassembly should be considered provided that they do not compromise assembly safety. Reuse and recycling of pinch clamps can be easy to imagine, but simple disconnection of tubing from hose barbs after use can be a challenging design task. The requirement to maintain a tight, sterile, leak-free connection during filling, draining, and pumping seems to be contrary to an easy-to-disassemble junction. Recyclability of plastics is improved by limiting their special-additives content. Use of natural (unpigmented) thermoplastics is desirable, which is already the case in most plastic parts used in biopharmaceutical applications.
Recycled Materials: The US Food and Drug Administration (FDA) has started to approve some mechanically recycled postconsumer plastics for food-contact applications. Apart from PET, few such materials are available on the market with the quality required for bioprocess applications. The extractables and leachables risk is still too great with recycled materials in fluid-contact components such as bag films and tubing. However, the risk is significantly less for packaging. With commitments of consumer-goods and food-industries leaders, we expect that the quality of recycled materials will improve, ultimately raising opportunities for their use in packaging of SU systems and components. The Bio-Process Systems Alliance (BPSA) encourages the SU industry to use recycled material wherever possible in items such as packaging and pallets. It would be a good practice also to note the percentage of recycled materials used for end-user awareness.
Plastics branded as “chemically recycled” already are available on the market (13). Those originating from chemical recycling have the same specifications as fossil-derived plastics and can be used in like-for-like applications. Polymers are manufactured from a mix of basic compounds originating from plastic waste or fossil raw materials, and the amount of recycled material is certified by mass balance. However, offerings are limited, and not all technical specifications can be fulfilled through the available portfolio of options.
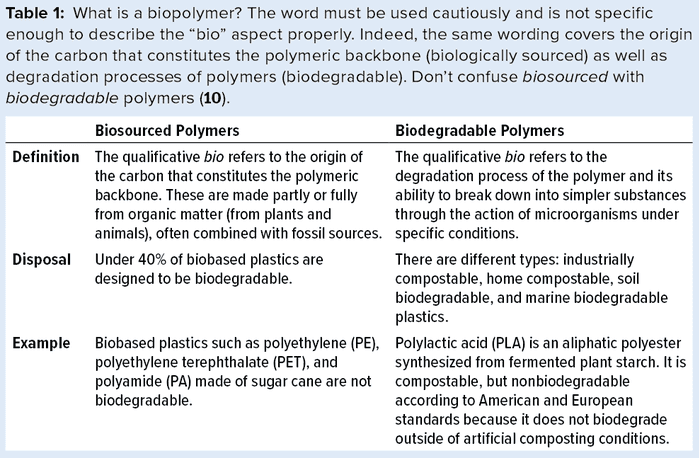
Polymers from Nonfossil Origins: Renewable feedstocks do not come from petroleum or coal. They include CO2 and methane captured through artificial carbon-capture and -use processes as well as biosourced feedstocks such as chemicals derived from sugar cane, corn, and other crops. Not to be confused with biodegradable polymers (Table 1), biobased materials are polymers, chemicals, and products made from biomass, biomass-derived byproducts, or CO2/methane derived from biological processes (also called organic recycling).
Biobased or partly biobased durable plastics including PE, PET, PVC, PC are technically equivalent to their fossil-based counterparts (14). PE made from biobased ethylene has been commercialized. A corn-sourced isosorbide can be used as a replacement for BPA monomer to manufacture a biobased polycarbonate. The first generation of biobased polymers was criticized because of a perceived conflict with food stocks; a second generation will come from plant-based monomer sources that are not food. The overall production capacity of biosourced polymers is estimated to be ~2 million tons, which represents <1% of global plastics production.
Biological methods are being developed to reprocess plastic waste, as well. Emerging technologies that use enzymatic processes to recycle plastic waste and manufacture new polymers have been demonstrated. These technologies currently are limited to specific polymers such as PET and have been run at pilot scale only. They are not yet viable commercially (15, 16). As for the integration of recycled polymers into SU systems, technical opportunities exist for integrating biosourced polymers into biopharmaceutical SU products and packaging. More effort is needed to explore the potential for putting recycled content — whether biosourced or traditional — into SU technologies. The portfolio will be limited until it has been demonstrated that all technical requirements can be fulfilled.
Economic Considerations: Because of the current low cost of oil, recycled and biosourced polymers today are more expensive than those based on virgin material originating from a fossil-fuel feedstock. Without other prioritization, that presents a significant barrier to adoption of alternatively sourced materials in SU systems and other applications. Integration of biosourced and recycled materials often must be coupled with a product or packaging redesign to offset the raw-material cost increase. This presents an opportunity to “rethink” product design, but new designs and new materials require change-control and validation work by users. Validating any change to an established bioprocess can be a significant burden, and changes always come with risk. Therefore, using recycled materials in packaging probably is the best short-term implementation opportunity for SU manufacturers.
Circularity at Point of Use
The principles of circularity should be applied across an entire biomanufacturing process, with a number of strategies available. In particular, a significant number of “reduce” and “reuse” opportunities arise in different stages. A monoclonal antibody (MAb) process using a SU manufacturing train at 2,000-L bioreactor scale can produce as much as 1.5–2.0 metric tons of plastic waste per 14-day batch (2). Below are some examples to illustrate how to reduce the amount of SU waste for disposal per batch.
Reduce and Reuse SUT Waste: Both end-users and suppliers can play a part in SU waste reduction. Suppliers can use engineered packaging to reduce the mass of films and cardboard, and they should strive to remove unnecessary layers (e.g., overbag films and foams) in secondary packaging. That would reduce the amount of material consumed and save on transport fuel, storage space/handling requirements, and discard/disposal activities. End users can discard fewer expired, unused materials by validating extended shelf lives and applying proper inventory management, such as first-in–first-out (FIFO) accounting. Users also can reduce the amount of unused material by implementing appropriate rejection standards to accept more functional material; through proper handling procedures on the operations floor (e.g., using appropriate cutting tools for secondary packaging to prevent damaging product bags); and by using optimal component design (e.g., tubing lengths) and specifications to prevent errant manufacturing.
Finally, users are encouraged to include environmental sustainability-based evaluation of their assembly designs. Removal of extra features such as unnecessary sampling ports could save on material, cost, and waste — making certain that the possibility of needing “extra” features is worth the waste and cost. Standardization reduces waste from expired products and excess packaging. When the same product is used for multiple applications, limited scannable stock-keeping unit (SKU) barcodes, safety stock, and bulk packaging all contribute to efficient and sustainable operations.
SU suppliers qualify their products for one use. However, some users might be able to validate reuse of certain products. The “Reusing SU Products” box suggests applications that could improve operational efficiency and waste minimization by reuse of SU components and systems.
Reduce Consumption of SU Components: Aside from those reuse opportunities, the biopharmaceutical industry is evolving to incorporate continuous-processing approaches that could help it achieve sustainability and improve process efficiencies.
Intensified bioprocessing methods such as continuous, integrated operations in smaller facility footprints could provide a way to reduce plastics use and thus improve a company’s environmental footprint. A fully continuous system would integrate upstream and downstream processes seamlessly to generate a constant flow of product. The advantages of integrated bioprocessing with minimal unit operations would be to decrease manual handling, improve safety, shorten processing times, and increase efficiency. Those efficiencies would increase the amount of total product being processed, giving manufacturing plants a reduced ecological footprint overall and providing for a favorable sustainability impact.
One example is perfusion processing. Using process-intensification methodologies has increased cell titers and help to reduce the amount of media and buffers used, effectively making more product with less raw material. Shortened cell-expansion times improve manufacturing networks and overall manufacturing timelines. Time savings also lower the cost of utilities such as electricity, thereby reducing process energy demands and fossil-fuel consumption for generating power. A standard, batch-mode cell culture process can take three to four weeks to run, whereas an intensified process can produce more material in less time (typically under three weeks).
Shortened process times would lessen the amount of energy used in heating, ventilation, and air conditioning (HVAC) systems as well. Those used to control air quality of the process environment can consume more energy than any other system in a manufacturing plant. Decreasing process times enables bioprocessors to make more product — or users can reduce facility airflow during extended downtimes and process-changeover periods. One benefit of using SU (closed) systems is that they allow cleanrooms to operate at lower classification levels, which provides operational savings. Those savings and sustainability benefits are compounded further when process time is reduced.
Although the intent is to reduce the costs of biomanufacturing by decreasing downtime between production lots and consequently raising productivity, continuous manufacturing has the added benefit of lowering the number of SU items and equipment required. That can reduce end-of-life waste when drug products are manufactured using continuous operations.
Circling Back
The new plastic economy movement and commitments to a circular economy from the consumer goods and food industries have created momentum in the plastics industry, with many innovation efforts focused on sustainability. That momentum is raising opportunities for enhancing the sustainability of SU products used in biomanufacturing. BPSA member companies are considering these opportunities carefully, along with examples from other industries, to improve the positive environmental impact of SU technology for bioprocesses. We encourage everyone involved with SU technology to work toward the goal of a circular economy.
SU material suppliers, integrators, and users increasingly are committed to sustainability as good social and business practice — which includes responsible management of used materials. BPSA endorses the study of SU sustainability along with implementation of new and improved operational technologies that will limit further the impact of these materials on the environment. The social benefits of SU technology currently overwhelm its residual environmental risks, and BPSA will keep working to reduce those risks further. SU technology is a good choice now, and through these efforts, will become an even better option in the future.
References
1 Barbaroux M, et al. The Green Imperative: Part One — Life-Cycle Assessment and Sustainability for Single-Use Technologies in the Biopharmaceutical Industry. BioProcess Int. 18(6) 2020: 12–19; https://bioprocessintl.com/manufacturing/single-use/the-green-imperative-part-one-life-cycle-assessment-postuse-processing-and-sustainability-for-single-use-technologies-in-the-biopharmaceutical-industry.
2 The New Plastics Economy: Rethinking the Future of Plastics. Neufeld L, et al., Eds. World Economic Forum: Geneva, Switzerland, January 2016; http://www3.weforum.org/docs/WEF_The_New_Plastics_Economy.pdf.
3 Smalley M. European Governments, Companies Sign European Plastics Pact. Recycling Today 9 March 2020; https://www.recyclingtoday.com/article/european-governments-companies-sign-plastics-pact-recycling-sustainability-2025-goals.
4 GVR-1-68038-823-7. Medical Plastics Market Size, Share, and Trends Analysis Report By Application (Medical Components, Wound Care, Cleanroom Supplies, BioPharm Devices, Mobility Aids, Tooth Implants), By Region, and Segment Forecasts, 2020–2027. Grand View Research: San Francisco, CA, June 2020; https://www.grandviewresearch.com/industry-analysis/medical-plastics-market.
5 Health-Care Waste. World Health Organization: Geneva, Switzerland, 8 February 2018; https://www.who.int/news-room/fact-sheets/detail/health-care-waste.
6 Single Use User Requirement Toolkit. Bio-Process Systems Alliance: Arlington, VA, 2017; https://bpsalliance.org/suur-resources.
7 Delaunay L, et al. How to Design and Qualify an Improved Film for Storage and Bioreactor Bags. Eibl R, Eibl D, Eds. John Wiley & Sons: Hoboken, NJ, 26 July 2019; https://doi.org/10.1002/9781119477891.ch19.
8 Hammond M, et al. Identification of a Leachable Compound Detrimental to Cell Growth in Single-Use Bioprocess Containers. PDA J. Pharm. Sci. Tech. 67(2) 2013: 123–134; https://doi.org/10.5731/pdajpst.2013.00905.
9 Jurkiewicz E, et al. Verification of a New Biocompatible Single-Use Film Formulation with Optimized Additive Content for Multiple Bioprocess Applications. Biotechnol. Progr. Early View 30 May 2014; https://doi.org/10.1002/btpr.1934.
10 Goldsberry C. Consumers Confused By Distinction Between Biobased and Biodegradable Plastics. Plastics Today 8 February 2020; https://www.plasticstoday.com/sustainability/consumers-confused-distinction-between-biobased-and-biodegradable-plastics/5040526662379.
11 Malloy RA. Plastic Part Design for Injection Molding: An Introduction. Second Edition. Hanser Publications: Cincinnati, OH, April 2010.
12 ASTM D7611/D7611M-20. Standard Practice for Coding Plastic Manufactured Articles for Resin Identification. ASTM International: West Conshohocken, PA, 2020; http://www.astm.org/cgi-bin/resolver.cgi?D7611D7611M.
13 Simon JM, Martin S. El Dorado of Chemical Recycling: State of Play and Policy Challenges. Zero Waste Europe: Brussels, Belgium, August 2019; https://circulareconomy.europa.eu/platform/sites/default/files/2019_08_29_zwe_study_chemical_recycling.pdf.
14 Wagner M. A Circular Economy for Plastics: Insights from Research and Innovation to Inform Policy and Funding Decisions. De Smet M, Linder M, Eds. European Commission: Brussels, Belgium, 2019.
15 Consortium to Support World’s First Enzymatic Technology for Plastics Recycling. Packaging Europe 8 May 2019; https://packagingeurope.com/to-support-the-world’s-first-enzymatic-technology-for-the-re.
16 Magnin A, et al. Enzymatic Recycling of Thermoplastic Polyurethanes: Synergistic Effect of an Esterase and an Amidase and Recovery of Building Blocks. Waste Manag. 85, 15 February 2019: 141–150; https://doi.org/10.1016/j.wasman.2018.12.024.
Magali Barbaroux is a corporate research fellow at Sartorius Stedim Biotech; Brian Horowski is director of process technologies at Wood Life Sciences; Sade Mokuolu is strategy implementation manager at Watson-Marlow Fluid Technology Group (a Spirax-Sarco Engineering company); Mark Petrich is both director of single-use engineering at Merck and first vice-chair of the Bio-Process Systems Alliance (BPSA); and Mitchell Snyder is applications engineering manager at Saint-Gobain Bioprocess Solutions. Corresponding author and BPI editorial advisor William Whitford is life science strategic solutions leader for DPS Group in Logan, UT; [email protected].
This paper represents conclusions of the BPSA sustainability subcommittee and not necessarily specific viewpoints of the companies represented by its members.
You May Also Like





