The Single-Use or Stainless Steel Decision Process: A CDMO PerspectiveThe Single-Use or Stainless Steel Decision Process: A CDMO Perspective
 Decisions regarding whether and when to use single-use (SU) (disposable) devices or stainless steel (SS) equipment for biopharmaceutical manufacturing have been discussed for more than a decade. To date, no argument in terms of safety, cost-effectiveness, or operational efficiency is fully convincing to choose one technology platform or the other for all applications. Biopharmaceutical companies often do not have in-use data to make strategic manufacturing decisions. But one group has been significantly growing its expertise in use of single-use technologies: contract development and manufacturing organizations (CDMOs). Their general offerings cover fee-for-service activities such as cell line development, process development, and drug substance and drug product manufacturing under good manufacturing practice (GMP).
Decisions regarding whether and when to use single-use (SU) (disposable) devices or stainless steel (SS) equipment for biopharmaceutical manufacturing have been discussed for more than a decade. To date, no argument in terms of safety, cost-effectiveness, or operational efficiency is fully convincing to choose one technology platform or the other for all applications. Biopharmaceutical companies often do not have in-use data to make strategic manufacturing decisions. But one group has been significantly growing its expertise in use of single-use technologies: contract development and manufacturing organizations (CDMOs). Their general offerings cover fee-for-service activities such as cell line development, process development, and drug substance and drug product manufacturing under good manufacturing practice (GMP).
CDMOs typically operate several manufacturing lines that differ in scale and type of equipment, and they can cultivate eukaryotic or prokaryotic cell types. Facility designs incorporate the use of traditional stainless steel skids, relatively new types of disposable equipment, or both side-by-side in a hybrid solution.
CDMO business models as service providers force them to look for flexible production capacity, fast campaign changeovers, and rapid production at different scales. Those demands are nicely fulfilled by the use of disposable equipment, which has made CDMOs early adopters of single-use technologies. Such companies have established underlying reasons for their equipment selection, as we discuss below.
Cost
To successfully implement single use and/or stainless steel manufacturing strategies, CDMOs must develop their own rationale to generate lower costs and streamline operations. Single-use systems offer many advantages (Table 1) over conventional stainless steel systems and have rightly gained wide acceptance in the biopharmaceutical industry. Advantages such as increases in batch success rate, elimination of potential cross contamination, more rapid changeover between campaigns, reductions in water and waste water requirements, and eliminations of
clean-in-place (CIP) and steam-in-place (SIP) validation all have been cited as reasons for using single-use systems (1). Although some advantages are no longer as strong as anticipated some years ago, the need to reduce project cost and time continues to be one primary driver for implementing disposable systems.
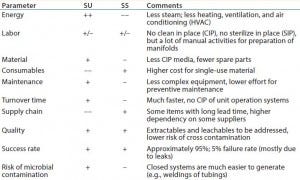
Table 1: Comparison of single-use (SU) and stainless steel (SS) systems concerning most important economical and quality-based parameters.
Implementing single-use systems reduces capital costs, but in some cases capital savings are offset by increased operating costs. Thus, life-cycle costs for many single-use applications are higher than for conventional stainless steel systems. Single-use technologies can be implemented through several potential entry points. For example, increasing manufacturing capacities can aim at supplying clinical material, serving market production, or address both opportunities.
The primary intention for single-use or stainless steel implementation will significantly influence different business cases. Unfortunately, there is neither a straightforward decision algorithm nor a simple yes-or-no answer to whether one solution should be preferred over the other. Some biopharmaceutical facilities use a mixture of stainless-steel and single-use systems. But what is the optimal mix of both when starting a campaign to expand manufacturing capacities? A thorough business analysis must be performed in all cases. Two methodologies can be applied:
cost of goods (CoG) analysis
life-cycle cost (LCC) analysis (or net present cost analysis)
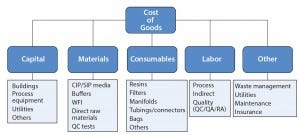
Figure 1: Cost of goods categories for a biopharmaceutical manufacturing process
Cost of goods manufactured is based on the amount of work-inprocess completed (Figure 1). This work-in-process includes costs of direct materials put into production plus direct labor and overhead. CoG is an important consideration in evaluating single-use systems and conventional stainless steel designs (2). Rentschler evaluated the distinct CoG for a virus filtration (VF) system based on stainless steel design and on single-use technologies (Figure 2).
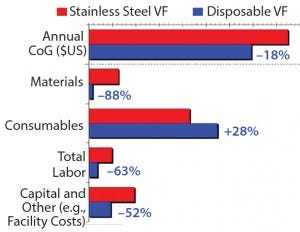
Figure 2: Annual cost breakdown of Rentschler’s stainless steel vs. disposable virus filtration system
CoG analysis is sufficient when comparing manufacturing costs of an existing process to decide future placement of that process for commercial supply in existing facilities. Rentschler performed a CoG analysis of whether to place an existing process to manufacture 100-kg drug substance in a 2,000-L single-use or in 3,000-L stainless steel facility. Even when taking into account all the advantages of the single-use technologies, the cost benefit in the end was on the side of the stainless steel facility. When setting up a “greenfield” plant for this specific process (including the corresponding infrastructure), the cost benefit is likely to remain on the side of the single-use based facility.
Understanding manufacturing process costs is not a trivial endeavor. Many important factors are not directly related to a process itself. Plant capacity, equipment depreciation, allocation, and other fixed costs can make cost estimation extremely difficult. An accurate evaluation includes expenses and a detailed process description, including facility and equipment depreciation. LCC analysis is a financial analysis technique that takes into account the total cash flows associated with a business scenario over its total lifetime.
LCC analysis recognizes the total cost associated with a facility, including construction capital and operating costs for a greenfield facility, assuming 10–20 years of operation. When properly applied, it is an excellent tool for evaluating business investment alternatives. When comparing options, the lowest LCC analysis result identifies the most favorable financial option.
Cost differentials also arise in two typical expansion scenarios:
new facilities based on modular design, including new infrastructure
reconstruction of existing facilities and adapting/expanding an existing infrastructure.
Single-use systems have a strong influence on a facility’s layout and can affect automation strategies, clean utility requirements, floor-to-floor heights, project timelines, procurement schedules, and even area classifications such as heating, ventilation, and air-conditioning (HVAC) design. Facilities that implement only single-use processing sometimes realize substantial advantages over facilities using conventional designs, but they tend to be limited in scale.
Scale
Upstream: Stainless steel upstream processing (USP) facilities can be customized to almost any size. However, typical large-scale steel bioreactors can be as large as 20 m³ and fermentors even get bigger for microbial processes. By contrast, single-use bioreactors are available to a maximum capacity of only 2 m³ today, with 5 m3 single-use bioreactors currently in development. Those size limits are mainly attributable to the achievable oxygen transfer. Low oxygen-transfer coefficients prohibit most microbial applications with an increased oxygen demand. So disposable bioreactors are primarily used for mammalian-cell cultivation. With regard to oxygen transfer, different impellers and spargers must be compared when final scale-up for market production leads to use of stainless steel bioreactors.
Using a perfusion process is one way to surmount the scale limitations of disposable systems. Although continuous USP processes have been applied for decades, they have recently gained significantly more interest. In such systems, product-containing cultivation media are constantly replaced by fresh media while a constant cell concentration is maintained within a bioreactor. That requires a cell-retention device that is based on either sedimentation or filtration.
Current centrifuges with disposable contact surfaces can handle up to 720 L/h (e.g., kSep 6000S from kSep Systems). Another fully disposable system is the alternating tangential-flow (ATF) technology (Repligen) that can deal with volume exchanges of up to 1,000 L per day. In such processes, a diaphragm pump delivers the cell-containing fluid through a hollow fiber that withholds cells. To prevent blocking the filter, a backflush is generated by temporarily moving the diaphragm in the opposite direction.
A second approach addressing scale limitations is process intensification. This strategy increases product titers in a smaller cultivation volume by improving media and feed compositions supporting high viable-cell integrals.
Downstream processing (DSP) also can benefit from the use of disposable equipment. In particular, two main unit operations must be considered: chromatography and filtration. Prime examples of single-use technologies are filters for bioburden or virus load (microfiltration) reduction, product concentration, and buffer exchange (ultra diafiltration) by tangential-flow filtration (TFF).
The scenario for chromatography is different. Although small-scale prepacked columns and membrane cartridges evolved simultaneously, it took a while until larger scale columns reached the market. Acceptance within the GMP community has grown, from 1% of the biopharmaceutical industry in 2010 to 37% in 2015 (3).
Despite commercial success of those columns, they still suffer from scale limitations. Columns from GE Healthcare Life Sciences (with the longest experience in preparing ReadyToProcess columns) deliver ≤20-L volume, representing a diameter of 35.9 cm. Opus columns from Repligen offer a more flexible alternative, allowing any resin to be packed in bed heights up to 30 cm and diameters of 60 cm maximum. Those scales are sufficient to handle harvest volumes of the biggest single-use bioreactors, making a fully disposable process now feasible (4). In the context of CMO operations, prepacked columns eliminate the need to pack and unpack the column. The investment in the column is fully chargeable to a client, so the column can easily be transferred back to a customer if a process needs to be established elsewhere.
Other essential equipment components that can be covered by disposable solutions are mixing tanks for conditioning intermediates and buffer supplies through plastic bags in containers. In those applications, scale limitations arise from end users’ requirements to move fully loaded containers from preparation and filling locations to final places where such components are used. In traditional stainless steel facilities, that is achieved by preinstalled hard piping. But the initial designs of piping and tanks limit flexibility of that facility in later processing. Manufacturers can gain greater flexibility by using mobile disposable equipment. But the upfront investment for movable container holders and the space they require should not be underestimated. Furthermore, an existing facility based on such equipment can be duplicated with significantly less effort and cost. That allows for closer localization to potential end users (e.g., for vaccine or biosimilar production) and simplifies relocation to commercially more attractive geographical areas.
Scale limitations for disposable processes are less crucial for indications that require less material to support fewer patients (e.g., for orphan diseases) or treatments with highly active molecules that require a lower-dose, less-frequent, or nonchronic administration. Not surprising, the first commercial process using disposables fully in USP was approved for an orphan disease. Shire implemented a successful combination of disposable equipment with a continuous process to manufacture Velaglucerase alfa as enzyme replacement therapy (ERT) in type 1 Gaucher disease (5).
Due to increasing titers in the biomanufacturing industry, production in disposable systems also might be sufficient for other, broader indications. Scale issues also exist for small volumes. For instance, in scale-down studies on virus removal or cell-cultivation optimization experiments, identical films in product-contact surfaces should be used independent of scale.
Safety
Discussion regarding process implementation either in a single-use or stainless steel facility should include more than factors such as scale, flexibility, contamination risk, and costs. Even though those topics often relate to process implementation in a single-use facility at small- or mid-size scale, considerations and concerns regarding overall safety are still of highest importance.
Biopharmaceutical manufacturing in a single-use facility is not simply a matter of risk-taking. Like manufacturing in stainless steel, bioprocessing is based on knowledge and on professional quality risk management according to regulatory guidelines (e.g., International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) Q9 quality documents). Risk assessments are integral tools for ensuring product safety, efficacy, consistency, and supply. Specifically, risk assessment of raw materials requires cross-functional inputs from all operative departments, including supply, development, manufacturing, and quality control and assurance. Difficulty showing an accurate science and risk-based assessment for the supply chain and for quality issues of single-use materials has led to concerns expressed throughout many industry discussions.
A lack of single-use standardization can lead manufacturers to single-source dependency. And single-use technology suppliers themselves are facing the same kind of dependency, because only a few sources exist for raw materials required to produce polymers used in many single-use components. The supply of raw materials — particularly those required for producing polymeric films used in bioreactors and flexible storage systems — is still a niche business. So it is important not only to include a supplier within a risk assessment, but also to work very closely together with that supplier because it is fundamental to have a continuous supply of product components that meet expected quality standards.
Accurate risk assessment requires consideration of quality issues for many single-use materials. Specifically, biomanufacturers should take into account variability of polymeric materials and related leaching of small compounds from polymers into products. Leachable components can have several sources: breakdown products of antioxidant additives, degradation products of polymers, processing additives, and residual monomers from incomplete polymerization (6). Quality risk assessment of leachables and extractables (components released under harsh conditions) has a primary focus on patient safety. Manufacturers must ensure that leachables that occur during processes will be removed from final products. So the closer to the final product a single-use material is used, the higher the risk of leachables contamination and insufficient elimination. Leachables from upstream processing have a rather low significance to patient safety because many downstream purification sequences have a highly sufficient capability of removing them.
With regard to process safety, however, leachables and extractables have come into focus, especially in regard to their impact on USP processes. Compounds leached from some materials have had detrimental effects on the proliferation of mammalian producer cell lines (7).
One study involved a comparative and systematic approach to testing different cell lines in different media (incubated before the study in different plastic bags) (8). Results revealed the need for urgent action toward testing and standardization of single-use materials. In another approach, end users from leading biopharmaceutical companies strongly suggested a generic extractable protocol for standardized testing of single-use materials under different conditions (9). These and other recommendations will certainly influence regulatory authorities and will flow into guidelines. They strongly support efforts to close the gaps of uncertainty that relate to the quality of single-use materials in biopharmaceutical manufacturing.
It is worthwhile to take a closer look on the practicalities of disposable systems. One reoccurring issue is the risk of leakage that could destroy a production batch. Such a situation can arise from improperly welded seams of bags, from punctures during handling, and from loose connections. Therefore, suppliers and end users need to provide a high level of quality control. A safe process also needs a well-established supply chain. Dependency on delivery timelines and storage space should be taken into account.
A further issue is the “quasimonopoly” position of some suppliers and the low leverage for negotiations on the side of end users. A final factor potentially limiting the broader acceptance and introduction of disposable systems is a lack of standardization and interchangeability of materials. For CDMOs, that lack of standardization can lead to unnecessary multiplications of systems when clients are willing to accept a number of single-use solutions.
Outlook
The most critical reasons to embrace single-use technologies are to reduce capital investment in facility and equipment and to increase speed to clinical trials. However, economic benefits of getting to market faster can dwarf cost of goods. So the impact of single-use systems on a project timeline must be considered when time to market is a significant driver. New stainless-steel–based facilities can take up to four years to build, validate, and become fully functional, which requires significant capital investment. Many emerging companies are looking for faster and less capital-intensive routes to drug production. They also may need to process multiple products in the same facility, which requires more efficiency and flexibility.
For a CDMO, the ability to respond to customer demands by offering different scales is crucial. The choice of scales also depends on market segment. If only drug substance supply for clinical phases 1 and 2 is needed, scales up to 2,000 L will be sufficient for most indications. But if late-stage material or even market supply is needed, then larger volumes might be required, which would require stainless steel and customized facilities.
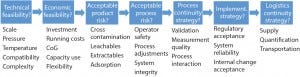
Figure 3: Decision process for single-use systems (10)
The speed of product changeover is another factor to consider. CDMOs with highly dynamic and frequently changing product portfolios benefit from disposables that allow faster transitions between different production campaigns. That leads to better facility use, faster returns on investment, and minimal unproductive downtimes for cleaning. Figure 3 shows general decision principles ranging from technical and financial feasibility to addressing product and process risks and integrating regulatory and supply aspects. A combination of three main factors — cost, scale, and safety — with the practical guidance in Figure 3 can help CDMOs make the right choice.
References
1 Whitford WG. Single-Use Systems as Principal Components in Bioproduction. Bioprocess Int. 08(12) 2010: 34–42.
2 Rogge P, et al. Economics of a Disposable Virus Filtration Process. Pharma Bio World 11(3) 2012: 26–28.
3 Langer E. 12th Annual Report and Survey of Biopharmaceutical Manufacturing Capacity and Production. Research and Markets: Dublin, Ireland. 2015.
4 Shukla A, Gottschalk U. Single-Use Disposable Technologies for Biopharmaceutical Manufacturing. Trends Biotechnol. 31(3) 2013: 147–154.
5 Press Release: Shire Announces European Approval of Manufacturing Facility For VPRIV® (Velaglucerase Alfa). Shire: Dublin, Ireland, 22 February 2012.
6 Jenke DR, et al. Materials in Manufacturing and Packaging Systems As Sources of Elemental Impurities in Packaged Drug Products: A Literature Review. PDA J. Pharm. Sci. Technol. 69 (1) 2015: 1–48.
7 Hammond M, et al. A Cytotoxic Leachable Compound from Single-Use Bioprocess Equipment That Causes Poor Cell Growth Performance. Biotechnol. Prog. 30(2) 2014: 332–337.
8 Eibl R, et al. Recommendation for Leachables Studies: Standardized Cell Culture Test for the Early Identification of Critical Films. Dechema Workgr: Frankfurt, Germany, 2014.
9 Ding W, et al. Standardized Extractables Testing Protocol for Single-Use Systems in Biomanufacturing. Pharm. Eng. 34(6) 2014: 1–11.
10 Technical Report 66: Application of SingleUse Systems in Pharmaceutical Manufacturing. Parenteral Drug Association: Bethesda, MD, 2014.
Corresponding author Peter Rogge is director of protein chemistry (peter.rogge@ rentschler.de). Dethardt Müller is senior director of process science, and Stefan R. Schmidt is vice president of process science and production at Rentschler Biotechnologie GmbH, Erwin-Renschler-Straße 21, 88471 Laupheim, Germany; www.rentschler.de.
You May Also Like





