- Sponsored Content
- Process Monitoring and Controls
Bioprocess Monitoring and Control: New and Continuing Needs in the Biopharmaceutical IndustryBioprocess Monitoring and Control: New and Continuing Needs in the Biopharmaceutical Industry
Sponsored by Waters Corporation
Equipment vendors, technology developers, and service providers have played an integral role in promoting innovation in the biopharmaceutical industry, from upstream production to final packaging and distribution of biological products. To enrich our understanding of the past 20 years of bioprocessing, BPI distributed questions to supplier companies. Below, Nick Pittman and Magnus Wetterhall of Waters Corporation reflect on advances in — and remaining opportunities for — process analytical technologies (PATs).
What Innovations Have Been Most Formative to the Past 20 Years of Biomanufacturing?
Wetterhall: Although biopharmaceutical manufacturing involves multiple interdependent processes, improvements to cell lines and culture techniques have been and will continue to be the primary drivers of bioprocess innovation. Reliable, genetically stable, and high-producing mammalian and bacterial cell lines have improved upstream production significantly, as have high-quality serum-free culture media and new options for flexible fed-batch, perfusion, and hybrid single-use bioreactor systems. Upstream gains have necessitated innovations in downstream purification, including the development of capture and polishing chromatography formats with increased capacity and selectivity and the introduction of rapid membrane-based solutions into connected and continuous manufacturing settings.
Such advances have driven the development and implementation of PATs, including novel sensors for in-line, on-line, and at-line monitoring of critical quality attributes and process parameters (CQAs, CPPs). Adoption of flexible, high-performing detection methods for multiattribute monitoring (e.g., mass spectrometry, MS) are expediting analytical turnaround times and enabling real-time release of drug products.
What Do You Hope the Biopharmaceutical Industry Will Achieve Next?
Pittman: The biopharmaceutical industry long has strived to accelerate development activities. Doing so will require improvements to clone selection and process development (PD). Currently, we have enabled process engineers to maximize yield, manufacturing efficiency, and drug-product quality, all while reducing development timelines. But we still need to integrate process monitoring, process control, and product-quality testing completely into manufacturing environments.
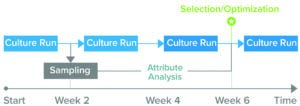
Figure 1: Because current analytics for upstream processes can take up to four weeks to generate results about product-quality and culture-media attributes, biomanufacturers might not make fully informed decisions before starting a culture run. (Attribute analysis accounts for both product quality and culture media.)
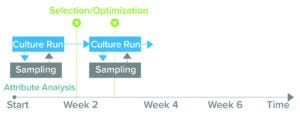
Figure 2: The BioAccord liquid chromatography–mass spectrometry (LC-MS) system generates product-quality and media-attribute data within 24 hours of sampling, enabling biomanufacturers to make informed decisions during cellculture processes and for future experiments. (Attribute analysis accounts for both product quality and culture media.)
Clone selection typically takes up to 10 weeks (Figure 1). Waters and Sartorius are collaborating to reduce that timeline by developing a solution, based on Sartorius’s Ambr multiparallel bioreactors and Waters’s small-footprint BioAccord system, that can analyze critical parameters for recombinant-protein production within 48 hours. The integrated system is designed to provide comprehensive information about product quality attributes and cell-culture media, in turn accelerating PD and reducing the number of requisite cell-culture runs from six to one or two (Figure 2).
Currently, core analytical groups use techniques such as MS to support their colleagues in PD. However, separating process and analytical functions can cause inefficiencies, and PD laboratories often lack direct access to MS instruments. Thus, they rely on other, often-busy laboratories to generate data. Data packages can be comprehensive, comprising many hundreds of measurements for multiple attributes, timepoints, and samples. Such data are acquired using complex, specialized equipment that requires both expertise and time, so each culture run can take two to six weeks or longer to generate the requisite data. Worries about analytical bottlenecks can compel biomanufacturers to measure fewer timepoints, assess fewer conditions, or accept long turnaround times.
What Developments Lie Ahead for Your Company and for the PAT Field?
Pittman: Misconceptions about MS have limited its adoption in biopharmaceutical manufacturing. Historically, mass spectrometers have been large, complex, and difficult to use. Sometimes that is still the case. But the BioAccord system enables nonspecialists to generate high-quality, high-confidence MS data with minimal training. Bioprocess engineers can take control of their own data and learn about what is happening in their cell cultures in near-real time. That is why Waters is working with partners such as Sartorius to develop applications for clone selection and upstream PD. Our goal is to help users adopt our solution seamlessly and then observe the direct benefits of simple, rapid, and dedicated analytical workflows.
Many companies are exploring advanced applications such as on-line feedback control in late-pilot or commercial environments. But adoption cycles can be long, and such solutions often require customization. Users can benefit more rapidly from their technologies by beginning early stage PD with standardized at-line solutions. Experience gained early on can facilitate implementation of advanced applications in late-stage production environments.
Our customers and collaborative partners also see significant potential in applying accessible MS instruments such as the BioAccord system to downstream applications. Near–real-time CQA data can inform operations from early PD stages all the way through manufacturing.
This is an exciting time, and we can enhance our at-line capabilities with further integration. Whether through automation of sample preparation, advances in sampling, or integration of existing technologies and solutions, the industry is poised to help scientists understand their cell-culture runs more quickly and easily than ever before.
How Might the Biopharmaceutical Industry Appear 10 or Even 20 Years from Now?
Wetterhall: We are witnessing a boom in both new and improved biological modalities based upon nucleic acids, viral vectors, and therapeutic cells and genes. Differentiation has increased among already established biologics, as has happened with the development of bispecific and trispecific monoclonal antibodies (bsAbs, tsAbs), conjugated monoclonal antibodies (MAbs), and fragments thereof. Such diversity is driving construction of modular and flexible manufacturing infrastructure — or renovation of existing facilities to accommodate that need. Demand is strong for multipurpose facilities that can be brought online easily. Biomanufacturers also are pivoting from processes with disparate unit operations toward connected and closed processes with integrated PATs.
The movement toward flexible multiproduct manufacturing raises both opportunities and difficulties. Drug makers will be able to produce personalized therapeutics or readjust manufacturing processes in response to events such as supply-chain disruptions and outbreaks of infectious diseases. But users of established bioprocess platforms might not be able to take advantage of such opportunities. For instance, it is highly likely that downstream purification formats will need to be developed for distinct modalities. The same is probably true for analytical instruments. Reliable and relevant in-process sampling solutions will be integrated with robust and fit-for-purpose MS solutions. There also is considerable need to implement integrated workflows that operators can handle easily, even in regulated manufacturing settings.
What Biomanufacturing Developments Have Surprised You the Most?
Wetterhall: The industry’s experience of the COVID-19 pandemic comes to mind. The biopharmaceutical industry and regulatory authorities worked together under accelerated timelines without compromising on patient safety, product quality, and appropriate documentation. New bioprocessing workflows were developed for mRNA-based vaccines. The success of Pfizer–BioNTech’s and Moderna’s respective mRNA products has opened the floodgates for other drugs based on nucleic acids.
Unlike traditional biomanufacturing, production of mRNA vaccines does not involve cellular systems. Enzyme-mediated transcription, encapsulation into lipid nanoparticles, and formulation of adjuvant constituents all will raise new obstacles for biomanufacturing, especially for process and product analyses. MS will become a critical method, not only for monitoring and controlling purity of raw materials, but also for following CQAs and CPPs throughout a workflow in real time.
As Nick said, these are inspiring times. We have just started to see the effects of exciting new modalities, workflows, and solutions in bioprocessing. We still have a bioprocessing “ocean” to explore.
Nick Pittman is marketing manager for global biopharmaceutical business, and Magnus Wetterhall is marketing manager for bioprocessing at Waters Corporation; [email protected]; [email protected].
You May Also Like






