- Sponsored Content
- Facility Design/Engineering
Challenging the Norms of Facility Design and InnovationChallenging the Norms of Facility Design and Innovation
August 19, 2022
Sponsored by Genesis
The past 20 years have spurred new technologies that enable flexible solutions to changing market demands. Small-scale tools, improved analytical methods, and innovative facility designs are among the notable breakthroughs. To enrich our understanding of the past 20 years of bioprocessing, BPI distributed questions to supplier companies. Below, Elyse Vlahos (director of process engineering at Genesis AEC) provides her perspective on innovation and future industry developments.
Innovation and Regulations
What have been the most important technical innovations over the past 20 years in bioprocessing? Facilities based on complex, large-scale stainless-steel bioreactors are evolving toward simpler sites with smaller-scale processes that can produce the same product volumes as traditional facilities within smaller footprints. Now with advanced therapy medicinal products (ATMPs) on the forefront, we see small-scale, patient-specific treatments with their own set of challenges. Such shifts have altered the trajectory of the industry.
The developments in science and on-line analytics have enabled dramatic increases in titer. Over 20 years ago, titers were in the range of 0.5 g/L. Now they are about 8 g/L. It’s easy to understand the implications of such a change. Twenty years ago, a 20,000-L bioreactor produced 10 kg of product: now a 2,000 L bioreactor can produce 16 kg of product (neither accounting for yield losses). That evolution directly influences the size and shape of facility designs. Continuous processes that harvest on a recurring basis can boost production even further.
Developments in analytics must be noted as well. Research and development innovations have enabled a better understanding of cell growth and production of desired proteins, and analytics are the tools that control such processes in real time. Instead of requiring personnel to pull samples, run tests, and respond to results, facilities are implementing automated control systems. Those systems continuously monitor key process parameters and address them immediately with programmed responses of additions and modification of gas-flow rates.
Coupling smaller batch volumes with single-use technologies has changed the look and complexity of facility designs. That has created shortened design phases and increased speed to market. Most important are the flexibility and interchangeable fit-out capabilities that result from single-use–based “plug-and-play” facilities. Product pipelines change based on clinical trial results, and the ability to repurpose a facility design quickly is invaluable.
The current climate is putting pressure on single-use component pricing and lead times, but that is not expected to persist. Instead, that pressure should drive others to compete in this market and open new possibilities. Understanding the history of the industry enables all of us to learn from and use similar approaches and techniques to negotiate the hurdles of current commercial-scale ATMP production.
What has been the most significant regulatory development in the past 20 years? The initiatives supporting “factory of the future” design concepts were monumental in challenging the way facility design is addressed. Amgen’s “next-generation” biomanufacturing facility in Singapore was the first of its kind, and it paved the way for others to follow. That design pushed the limits of readily accepted current good manufacturing practices (CGMPs) at that time while maintaining intent of the practices and integrity of products. Such concepts provided the impetus for regulatory guidelines (e.g., Pharma Inspection Cooperation Scheme Annex 2, 2017) to adapt to new proven methods of operation.
The essence of GMP is to protect a drug product. That will never change, but the methods used to accomplish the goal will continue to evolve. Originally GMP manufacturing was accomplished within a cleanroom environment — open processing in a Grade A biosafety cabinet (BSC) environment with a Grade B background. The push toward validating process closure led to discussions about how to operate more efficiently. Feeding media and buffer from outside a cleanroom and shrinking the classified spaces wouldn’t have been possible without that shift.
Such changes have led to discussions about how other areas can be improved. Here is where the history of biotechnology evolution comes into play. We’re at a juncture for ATMPs. Somehow it seems as though the industry is back to the past, with open operations in a BSC, working in environments such as clinics and hospitals, and variability in starting materials. We need to use the lessons learned and challenge those norms to support the latest scientific developments. The goal is to drive toward process closure; develop repeatable and flexible designs; and keep new challenges in mind, including patient access to new products.
The Next Inflection Point
How might your industry appear in five, 10, or even 20 years? The future industry will focus on cell and gene therapies, mRNA platforms, and lipid nanoparticle delivery methods. Those areas suggest cures and targeted treatments aimed at reducing the typical side effects observed with traditional therapeutic treatments. Opportunities for new ATMP entries into the market seem limitless based on the science. The rest of the biopharmaceutical industry remains in play in perpetuity, but ATMPs now are the monoclonal antibodies of the 2000s.
Such opportunities can bring inherent difficulties. Many advanced therapies are in the development pipeline, and each targets a relatively small patient populations. Until the industry creates cost-effective solutions, we haven’t addressed the obstacle of getting treatments to patients. Automated handling from receipt of apheresis/leukapheresis material to processing and filling infusion bags is needed. Advancements in robotic technology — currently in its infancy within bioprocessing — will have a huge influence on small-scale operations. Robotics and automation seem cost prohibitive. However, such costs should decrease with reproduction. Although the first fully automated cell-therapy suite developed for commercial production will incur substantial up-front cost, repeating that design should improve efficiencies, and reusing that design as a platform for other therapies will be possible with modifications to procedures and recipes.
Other fundamental challenges include chain of custody and patient access to therapies with limited storage and processing times of patient material and ultimately the product made from it. How other industries have approached these issues can provide insights. How can the biopharmaceutical industry apply distribution center strategies to repeatable cell processing hubs that can be deployed on demand where needed while tracking patient material throughout a process? We are part of an exciting time in the biopharmaceutical industry, and we are able to craft the solutions that will drive the next inflection point.
What advice do you have for new life science professionals? I challenge the next generation to become well-rounded experts, to trust yourselves, and to recognize your value. Hone your skills in your area of expertise and be curious about what motivates the people with whom you interact. Be passionate about understanding your field better than anyone else — not only your area of focus, but your field, including people affected by your work; those who contribute to your work; and those who interact with your work and their drivers, needs, and goals. Be the process engineer who thinks like a regulatory expert. Be the architect who thinks like a process engineer. Be the designer who thinks like an operator. Understanding what drives a project, what drives others, and how it all interconnects will help you to be a better solution provider, in whatever part of the biotechnology industry you’re in.
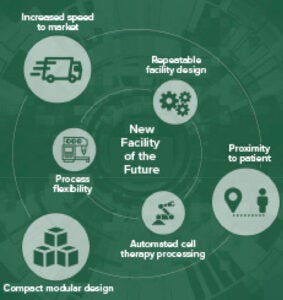
Figure 1: Concepts of a future facility
Facility designers aim to understand the interconnectivity of projects, a design’s relationship to all disciplines involved, and the influence that has on end users. That is where cohesive, coordinated solutions come into play. This is where you can push yourself and your team to deliver like no one else. Trusting your instincts will enable you to provide sound recommendations in the best interest of a project — even when it is not in sync with a desired path forward. Push yourself to recognize your own value through any self-doubt. That builds confidence that readily shows. Those are key aspects of building long-term trust and relationships with clients, service providers, and within your own organization.
What lies ahead for you and your company? I’m part of a team that shares this “extension of thinking” approach toward design, in which you consider the drivers for others on the project to execute in a way that is deliberate. That is where magic happens — big ideas, pushing industry norms, and considering efficiencies and lessons learned through shifts in regulations to reach the next level in design. The factories of the future might look like small, repeatable modules that can be installed and validated quickly to address supply-chain efficiencies from patient to process and then back to patient. Such designs will enable speedy implementations and flexible changeovers. They will address the newest technologies and maintain system closures. In turn, those designs will be able to handle a range of processes needed to address the myriad afflictions, deficiencies, and diseases that patients face.
I am energized by the prospects ahead on this next wave of revolution in our industry, which will be driven by the mission we all share to help patients obtain life-altering treatments.
Elyse Vlahos is director of process engineering at Genesis AEC, One Sentry Parkway, Suite 100, Blue Bell, PA, 19422; [email protected].
You May Also Like






