- Sponsored Content
Control of Critical Process Parameters Using In-Situ AnalyticsControl of Critical Process Parameters Using In-Situ Analytics
December 11, 2017
Sponsored Content
 Biopharmaceutical companies can develop entire end-to-end single-use production platforms and use them for commercial manufacture of their biological products. Single-use facilities are flexible, can be implemented quickly, and do not require the large up-front capital investments needed for stainless steel equivalents. However, single-use facilities must be supplied with a large quantity of high-quality consumables. Biomanufacturers should pay close attention to their supply chains for those consumables to ensure that they are robust, integral, and fully compatible with biological expression systems and products.
Biopharmaceutical companies can develop entire end-to-end single-use production platforms and use them for commercial manufacture of their biological products. Single-use facilities are flexible, can be implemented quickly, and do not require the large up-front capital investments needed for stainless steel equivalents. However, single-use facilities must be supplied with a large quantity of high-quality consumables. Biomanufacturers should pay close attention to their supply chains for those consumables to ensure that they are robust, integral, and fully compatible with biological expression systems and products.
Simply attempting to ensure that single-use manufacturing platforms provide equivalent performance to stainless steel facilities is a missed opportunity. Innovations in the fields of process analytical technologies (PAT) and data analytics allow engineers to gain greater understanding and control of single-use processes. Those innovations deliver the combined benefits of more consistent production of high-quality product with improved productivity. Here I explore innovative PAT tools for control of critical process parameters (CPPs).
Scientists and engineers can apply a number of methods for understanding conditions within bioprocesses. The approach a company adopts should reflect the context in which it wishes to implement a given analytical method. For example, off-line multiparameter analyzers can be linked to autosampling systems and provide significant insight into conditions inside bioreactors. Such a set-up is particularly useful during process development, when sampling and analysis often cause bottlenecks that reduce a company’s ability to reach the clinic quickly. Autosampling and off-line analysis can be used to monitor nutrients and metabolites, automate cell counts, and provide some level of nutrient feed control.
Sartorius Stedim Biotech has integrated the Bioprofile® FLEX2 from Nova® Biomedical into its ambr® 15 automated, high-throughput microbioreactor system. This allows customers to monitor multiple process parameters with cell cultures during design of experiment (DoE) studies and obtain data every six hours. Such an approach works well in laboratories but is difficult to implement into good manufacturing practice (GMP) environments because of the complexity associated with running these systems without increasing process risk. Furthermore, process parameters are not being monitored between samples, which limits the degree to which a process can be controlled and automated successfully. For example, controlling glucose at a concentration <1 g/L would be unachievable with this type of approach for the duration of a batch.
Bioprocess engineers historically have made good use of “soft sensors” such as glucose uptake rate, biocapacitance-based glucose control, and pH-based feed control to automate equipment even in GMP settings. Soft sensors provide an indirect, inferential, and predictive method of monitoring cell cultures when no direct method for measuring a specific parameter currently exists. These can provide visibility of a process to operators and allow some limited control. Soft sensors are particularly useful for verification of more advanced analytical approaches, which can be important in developing automation strategies. The disadvantage of soft sensors is that the models they rely on to estimate process outputs often are highly complex and susceptible to process variation.
There is considerable room for innovation in bioprocess PAT technologies that would overcome the limitations of off-line analyzers and soft sensors in GMP settings. Sartorius Stedim Biotech believes that the best way to control CPPs is to measure them directly and integrate such methods into equipment control systems with smart feedback algorithms for automated control loops. Bioprocess engineers need to scale and adapt technologies that are fully capable of measuring CPPs to all necessary bioreactor formats. New PAT tools should provide real-time sensing capability with an in situ analyzer that can provide rapid feedback and maximum control. They must be installed into single-use systems without breaching integrity and causing system sterility loss. Finally, the integration of such sensors into plantwide control systems or data historians should be as simple as possible for customers through plug-and-play technologies.
Sartorius Stedim Biotech has developed two new PAT technologies that meet those criteria: The BioPAT® Trace allows on-line measurement and control of glucose and lactate, and the BioPAT® Viamass capacitance probe measures viable biomass within cultures.
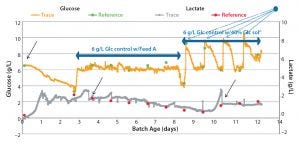
Figure 1: Comparing BioPAT® Trace data collected in-situ with off-line glucose and lactate measurements; blue dot points to
fixed-volume bolus dosages of Feed A.
BioPAT® Trace: Controlling glucose and lactate levels in bioreactors maintains the health of cell cultures and ensures desired glycosylation profiles of biopharmaceutical products. Figure 1 compares BioPAT® Trace data collected in-situ with off-line glucose and lactate measurements. Companies can use the BioPAT® Trace with 500-mL to 100,000-L single-use or stainless steel bioreactors based on rocking motion or stirred-tank designs. It can allow nearly constant glucose feeding at concentrations <0.5 g/L. Control in that range is ideal for perfusion cell cultures, in which excessive glucose availability can lead to efficiency loss and diminished yields. Investigators at Biogen Idec have increased product titers by >30% with a fed-batch Chinese hamster ovary (CHO) cell culture process by installing a BioPAT® Trace with automated feedback control into 3-L glass bioreactors. The process had given low yields previously because of high lactate levels. The BioPAT® Trace monitored glucose and lactate concentrations every 15 minutes, a frequency of sampling that was not possible using an off-line analyzer, with which sample-volume requirements would have been prohibitive at such a small scale. The BioPAT® Trace provided more responsive control than would have been possible with a pH-based strategy because the medium buffer delays the pH response to lactate change, making it difficult to control around a lactate set-point.
Researchers at the Massachusetts Institute of Technology (MIT) scaled up an FRhK-4 roller-bottle culture to a microcarrier culture perfusion system using a BIOSTAT® B Univessel benchtop bioreactor from Sartorius Stedim Biotech. They used a BioPAT® Trace for the real-time monitoring of glucose concentration. Spent media were removed from the culture once glucose concentrations dropped <1 g/L. A level probe detected the resulting loss in volume and initiated feeding of the culture with fresh media.
BioPAT® Viamass: Viable biomass is a parameter that cell culture specialists use to visualize the trajectory of cell growth. It allows operators to monitor processes and detect deviations from an expected batch trajectory. Biomass levels also can be used to control feed addition, monitor and control perfusion cultures, and detect the optimum point for harvesting bioreactors. For downstream processing, engineers can use biomass levels in their bioreactors to size clarification technologies such as diatomaceous earth filtration.
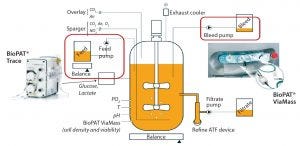
Figure 2: Enhanced control of a perfusion culture with the BioPAT® Trace and BioPAT® Viamass
The BioPAT® Viamass works with a broad range of bioreactor scales and formats. It helps engineers improve the productivity of their bioprocesses by controlling bioreactor bleeding when implementing continuous bioprocessing. A control loop limits cell densities to a desired value and prevents overgrowth. A peristaltic pump removes cells through a dip tube. An increase in viable cell density, as measured by the BioPAT® Viamass, defines the flow rate at which that occurs. For enhanced control of perfusion cultures, engineers can install both a BioPAT® Trace and BioPAT® Viamass for controlling feed pumps and bleed pumps together (Figure 2).
Conclusion
Here, I have shown how bioprocess engineers can improve control of their bioreactors using dedicated, real-time, on-line sensors. These will allow the industry to develop more controlled and automated single-use platforms that deliver consistent product quality and improve productivity. Novel PAT tools set the foundation for advanced process control strategies using multivariate data analysis. The next article explores this subject in greater detail. •
Dan Kopec is technology expert in process analytics at Sartorius Stedim North America Inc.
You May Also Like






