- Sponsored Content
Creating Value As a CDMOCreating Value As a CDMO
November 16, 2023
Sponsored by Aton Biotech
As a contract development and manufacturing organization (CDMO), Aton Biotech provides a comprehensive set of services to clients developing biological drugs. Those services range from cell line development to clinical development and end-to-end commercial manufacturing.
A Long History
Aton emerged from a biopharmaceutical company called Henlius that was established in Shanghai, China, nearly 13 years ago. Today, Henlius has five products on the market including rituximab, trastuzumab, and adalimumab. From the beginning, company leadership decided to build internal manufacturing capacity to strike a balance between quality and efficiency.
In 2022, a team of experts spun out from Henlius to form Aton Biotech, which is now a separate legal entity. Aton fully leverages profound experience derived from Henlius in providing end-to-end services to other biopharmaceutical companies. We felt confident that we not only could replicate the success that Henlius has experienced, but also would establish a CDMO that stands apart from others in the value it can create for clients. To date, we have produced >700 commercial batches and >130 clinical and toxicology batches in working with more than 30 molecules. Our facilities have been audited successfully by regulators and partners more than 90 times.
So although Aton Biotech may be a new name among CDMOs, we have a proven track record of success in delivering unmatched value for clients. We blend the nimbleness and flexibility of a startup with the expertise and experience of a long-standing CDMO. That provides our clients with confidence when they partner with us — whether their programs are in preclinical development or transferring to us for commercial supply.
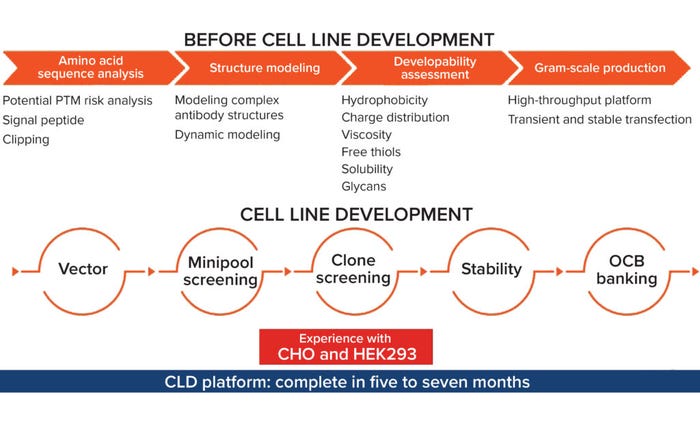
Figure 1: Aton Biotech’s cell-line development platform; (PTM = posttranslational modification, OCB = operating cell bank).
Generating Value
We focus on adding value for each client and derisking programs. Our clients face many different challenges, and that’s where our experience and ability to be nimble and flexible are important. First we seek to understand their specific needs; based on that, we extrapolate the value that can be generated. The solution is never “one size fits all” because value comes in many different forms depending on molecule type, life-cycle stage, and so on.
For some clients, value may derive from our fully integrated services presenting opportunities to get creative with deal structures, move faster, and reduce risk with a simplified supply chain. Many CDMOs claim to be “end-to-end” but actually partner with other organizations to create the perception of a complete solution. You can’t derisk a process and gain the same levels of efficiencies with that approach.
Many clients in the preclinical phase often have simply licensed a molecule from an academic laboratory and need to move quickly to develop a data package that will support an investigational new drug (IND) filing and help raise funds. We can meet aggressive timelines within a competitive cost structure by leveraging our preclinical development platform.
Our complete development platform includes cutting-edge biopharmaceutical production technologies and analytical methods. We also have a proprietary cell line that can be evaluated along with other, commercially available cell lines, allowing us to find the best solution for each client. That capability has also been verified in commercial production of Henlius products.
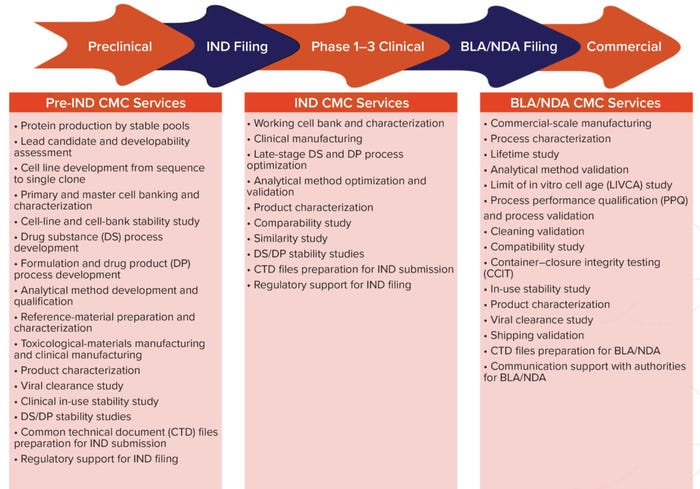
Figure 2: End-to-end chemistry, manufacturing, and controls (CMC) platforms; IND = investigational new drug, BLA = biologics license application, NDA = new drug application.
Other Services Offered: We provide services that can take a company from preclinical development all the way to commercial production. We offer chemistry manufacturing and controls (CMC) services to support IND, biologic license, and new drug application (BLA/NDA) filings. Companies entering and advancing through clinical development can work with us to address their requirements for success: process optimization, product characterization, stability studies, and (for biosimilars) comparability studies. Regulatory and submission support are essential, and we have a team of experts who support and counsel clients in those mission-critical aspects of drug development. Our CMC services for BLAs/NDAs are comprehensive and include final fill–finish. Many clients come to us in the later stages of development, and our highly skilled team can ensure successful technology transfer.
Biologics Supported: Clients come to us with all types of biologics. Our team has experience with both conventional and unconventional proteins, including monoclonal antibodies (MAbs), fusion proteins, multispecific antibodies, antibody–drug conjugates (ADCs), and enzymes.

Figure 3: Antibody–drug conjugate (ADC) plant overview — fully integrated drug-substance and drug-product manufacturing.
We have secured several global collaborations, including one with KangaBio for development and manufacturing prodrugs of immune-stimulating agents and other multispecific biologics to eliminate toxicities and increase drug efficacy (1). Aton will provide end-to-end services including cell line development, cell-bank construction and verification, process development and optimization, analytical method development, process transfer and confirmation, clinical manufacturing, commercial-scale manufacturing, stability studies, and IND and NDA filing support.
ADCs are an example of where we can deliver significant value for our clients and derisk their projects and processes. By leveraging the Henlius ADC facility, Aton has drug-substance and drug-product manufacturing capabilities to produce antibodies and conjugate them to payloads, then perform the fill–finish steps, all in one location with full quality assurance and control (QA/QC) capabilities. This setup simplifies what otherwise would be a highly complex supply chain, thus shortening time to market.
Customer-Centric Mindset
I’ve been in the life sciences for over 20 years, mostly with CDMOs around the world: DSM, Samsung Biologics, Catalent, Abzena, and WuXi. My experience with those organizations helped me see what was good and what could be optimized, and also helped me to crystallize a customer-centric mindset in establishing Aton Biotech as a world-class, value-creating CDMO.
As with all CDMOs, quality is a must — and it is at the forefront of Aton Biotech. Beyond that, I strategize about how we create ultimate value to alleviate our clients’ pressure points, including concerns about risk profile and flexibility. It is important to build a strategic partnership that can achieve maximum value. We determine how best to align our offering with our clients’ needs so that we can achieve long-term partnership.
Ultimately, Aton Biotech is a service provider. So it is our job always to ensure successful outcomes to the best of our ability for all our clients. We always have our customers at the top of our minds.
Reference
1 KangaBio and Aton Biotech Enter Into Development and Manufacturing Strategic Collaboration. Cision 20 December 2022; https://www.prweb.com/releases/2022/12/prweb19082312.htm.
Min Park is chief business officer at Aton Biotech, Floor 6, Building B, Number 1289 Yishan Road, Xuhui District, Shanghai 200233, China; 021-33395966; https://www.atonbio.com. For more information, see bit.ly/3U9xj3i.
You May Also Like






