- Sponsored Content
- Gene Therapies
Effective Processing of Lentiviral Vectors: Introducing a Paradigm-Shifting TechnologyEffective Processing of Lentiviral Vectors: Introducing a Paradigm-Shifting Technology
Sponsored by Astrea Bioseparations
Therapeutic developers face significant challenges in purifying cell and gene therapies (CGTs). Current technologies for laboratory-scale lentivirus (LV) feedstock preparation are inefficient and not fit for purpose. From the benchtop to the clinic, all stages of CGT development require new solutions that break away from the current paradigm of biopharmaceutical manufacturing.
Nereus LentiHERO nanofiber-based technology can address difficulties associated with purifying large and fragile modalities. Here, experts from the viral vector team at Astrea Bioseparations discuss how Nereus LentiHERO technology enables laboratory-scale lentiviral vector (LVV) purification by addressing industry needs for increased throughput and improved viral particle recovery.
Special Considerations for Lentivirus
How do LV characteristics influence their purification?
Ian Scanlon (viral vector downstream processing): LVVs differ in several respects from other viral vectors used in CGT. Whereas adenoassociated virus (AAV) capsids, at 25 nm in diameter, are similar in size to large proteins, LVVs are considerably larger at 80–100 nm. The larger size of an LVV is an advantage because it enables incorporation of genetic constructs up to 10 kb. However, LVVs are too large for traditional, resin-based chromatography methods to achieve sufficient capacity. Thus, work with LVVs necessitates different adsorbents for purification.
LVVs also tend to be more unstable than nonenveloped viruses such as AAV. The LVV envelope plays an important role in vector functionality, including transduction and tropism. But some processing conditions can diminish functionality, including changes in temperature, pH, and ionic strength of bioprocessing fluids.
Sujeong Yang (viral vector production): Host cell proteins (HCPs) are major contaminants that can cause undesirable immunogenic effects. The similarity of LVVs and contaminants can cause difficulties for manufacturing a pure LVV product.
What is the Nereus LentiHERO solution, and what advantages could it raise for LVV purification?
Daniella Steel (product management): Developers require significant improvements over legacy solutions, with novel adsorbents that provide large molecules with ready access to considerably more binding surface area without increasing processing time.
The Nereus LentiHERO solution is based on proprietary AstreAdept nanofiber material. The nanofibers are electrospun, making the surface area highly accessible to viral vectors and enabling significantly faster flow rates compared with those for traditional bead- or membrane-based adsorbents (Figure 1). Consequently, AstreAdept separation technology can reduce residence times to less than one second, decreasing processing times for fragile modalities.
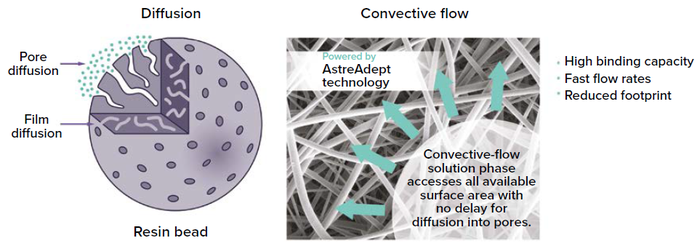
Figure 1: Readily accessible high binding surface area enables more effective processing of lentiviral vectors.
Increased LVV capture is essential to maximizing LVV production for viral vector development. Functionalized AstreAdept nanofibers provide dynamic binding capacities of 1.6 × 1011 LV particles/mL of adsorbent, enabling users to recover more from their current feedstock volumes and, in turn, reducing pressure to expand upstream production.
AstreAdept technology has been incorporated into the easy-to-use Nereus LentiHERO spin-column format to bring the advantages of functionalized nanofibers to viral vector purification at laboratory scale. Further development of AstreAdept technology is underway to address needs for large-volume bioseparation processes, including for process-development, pilot, and manufacturing scales in CGT applications.
Improving Laboratory-Scale Lentivirus Purification
What limitations come with existing laboratory-scale purification approaches, and how can Nereus LentiHERO technology help to address them?
Yang: Ultracentrifugation and density-gradient ultracentrifugation can purify and concentrate LVVs, and both are frequently used methods for laboratory-scale vector preparation. But they lack scalability, require high-powered centrifuges and special operational expertise, and consume considerable time. Ultimately, throughput is limited.
Scanlon: There is no standard downstream purification process for small-volume applications. Molecular-weight cutoff (MWCO) filters can be used with benchtop centrifuges to concentrate clarified feedstocks, but such filters have limited capability for removing impurities. The same considerations apply for tangential-flow filtration (TFF), although LVVs also are concentrated along with salts, proteins, and DNA — generally for extended periods because of the time required for concentration.
Precipitation sometimes is used to remove impurities selectively. PEGylation of vectors can enable selective product concentration, for instance. The problem here is that, once again, adding unit operations to an LVV process typically reduces the final yield of product considerably.
Alternatively, ion-exchange membrane adsorbers can be applied to align a low-volume purification process with chromatography steps that often are used during clinical-scale LVV manufacturing. However, functional product recovery is compromised using high-salt elution steps.
Steel: There is a clear need for a process that increases sample-preparation throughput while enhancing sample purification and reducing product loss. In addition to the superior capture characteristics of the underlying nanofiber technology, the Nereus LentiHERO solution enables gentle processing, including low centrifugal speed, reduced processing times, and mild elution conditions that enable high LVV recovery. Nereus LentiHERO technology achieves yields of 60% of loaded LV particles, which is a significant improvement over the 15–30% recoveries observed with other methods. The improvement comes from anion-exchange chromatography, which often is applied during LVV manufacturing. Consequently, process development time required to help a therapeutic asset advance through the clinical pipeline is reduced.
Minimizing HCPs is important for limiting immunogenicity, even at laboratory scale. The Nereus LentiHERO solution can reduce HCP contamination of LVV feedstocks by 95% and decrease requisite feedstock volumes by as much as tenfold depending on loading volume. Reducing both contaminant levels and sample volumes facilitates subsequent concentration.
Because Nereus LentiHERO technology comes in a spin-column format, sample throughput can be increased easily by scaling out through maximizing benchtop centrifuge capacity. By incorporating the Nereus LentiHERO solution into LVV feedstock preparation, multiple samples can be processed simultaneously, and bottlenecks can be reduced.
There is a pressing need to address laboratory-scale issues with LVV purification. A time-efficient process that simultaneously increases recovery and enhances purification would be an enabling technology for future LVV-based therapy development.
The Nereus LentiHERO solution for LVV development and production laboratories is designed to increase sample-processing throughput and improve upon LV particle recoveries. This nanofiber-based technology empowers therapeutic developers with tools that they need to purify the quantities that they desire, ultimately helping the CGT industry to bring therapies to patients faster than ever before.
Ian Scanlon is a subject matter expert in viral vector downstream processing, Sujeong Yang is a subject matter in viral vector production, and Daniella Steel is a senior product manager for cell and gene therapies, all at Astrea Bioseparations, Horizon Park, Barton Road, Comberton, Cambridge CB23 7AJ, UK; 44-0-1223-433-800; [email protected].
You May Also Like






