- Sponsored Content
Enhanced Assurance of Supply for Single-Use Bags: New Flexsafe Bag FamilyEnhanced Assurance of Supply for Single-Use Bags: New Flexsafe Bag Family
August 15, 2014
 Growing adoption of single-use bags for the production of biopharmaceutical drugs raises new challenges, including consistent product quality, improved assurance of supply, robust change management, and business continuity planning. In close collaboration with resin and film suppliers, Sartorius Stedim Biotech’s polymer scientists and biologists have followed a quality by design (QbD) program for the development of a completely new polyethylene film S80 thus achieving consistent performance of the new Flexsafe bags for all bioprocess steps and applications. The partnerships and agreements with our suppliers, the complete understanding and control of our manufacturing process from the resin, and the film extrusion to the final sterile bioprocessing bag are the prerequisites to ensure reliability of our supply chain. This assurance of supply relies on a long-term contract with our film supplier. In addition, the establishment of resin specification instead of general trademarks provides robust change control and facilitates change management because we can rapidly validate an equivalent resin in the case of a discontinuation of a raw material.
Growing adoption of single-use bags for the production of biopharmaceutical drugs raises new challenges, including consistent product quality, improved assurance of supply, robust change management, and business continuity planning. In close collaboration with resin and film suppliers, Sartorius Stedim Biotech’s polymer scientists and biologists have followed a quality by design (QbD) program for the development of a completely new polyethylene film S80 thus achieving consistent performance of the new Flexsafe bags for all bioprocess steps and applications. The partnerships and agreements with our suppliers, the complete understanding and control of our manufacturing process from the resin, and the film extrusion to the final sterile bioprocessing bag are the prerequisites to ensure reliability of our supply chain. This assurance of supply relies on a long-term contract with our film supplier. In addition, the establishment of resin specification instead of general trademarks provides robust change control and facilitates change management because we can rapidly validate an equivalent resin in the case of a discontinuation of a raw material.
Business continuity planning is finally addressed with a mix of already available redundant equipment and manufacturing locations, development of backup equipment, and maintenance of safety stocks for resins and film rolls.
A Partnership, Material Science, and Quality by Design Approach
To meet current industry challenges for single-use and a more robust product quality, a new proprietary PE S80 film and Flexsafe bag family have been developed in partnership with Sdpack, our film manufacturer. With Sdpack, we have established direct contacts and trust with selected industry leading suppliers of resins, thereby providing us with an unprecedented level of information about the resin and additive package formulation as a key part of our new strategy to achieve complete control of our entire supply chain, from raw materials to finished products.
This partnership with Sdpack, combined with material science and QbD expertise, allows us to achieve complete understanding and control of our manufacturing process, from the raw materials to the final bioprocessing bags.
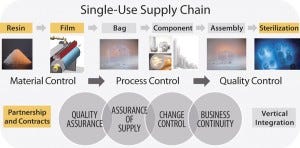
FIGURE 1: Overall supply chain for single-use bioprocessing bags
Proceeding with the selection of several raw materials and several film compositions, we have established the formulation, the additive package, and the film specifications within a design space to make Flexsafe bags suitable and scalable for all upstream, downstream, and aseptic bioprocessing steps.
Complexity of the Supply Chain
The assurance of supply for our Flexsafe bags is guaranteed by our long-term supply contracts established with our different resin suppliers and with Sdpack. We have a 10-year supply agreement in place for the S80 PE film and a last-time buy option for a minimum of two years of resin demand in case of a change or discontinuation. With Sdpack, we also benefit from the large state-of-the-art film-extrusion capacity that is required to sustain the demand and double-digit growth of single-use bioprocessing bags every year. Here again the complete control of our supply chain and of our manufacturing processes, from the raw materials to the final assembly, is critical to offer both quality and assurance of supply.
Robust change control is achieved thanks to our long-term contracts with resin and film suppliers, the establishment of resin and film specifications, and finally, the film-extrusion specifications within a defined design space.
The first long-term contract includes a six-month notification for the resin and a two-year notification for the film, should there be changes in the raw materials and/or the manufacturing processes. In addition, we have a last-buy option of unchanged material for two years of resin demand.

TABLE 1: Global approach to quality, assurance of supply, change control, and business continuity
Even more important, the establishment of resin specifications instead of general trademarks provides robust resin change control and facilitates change management. With the established specifications and the understanding of the critical quality attributes for the resin, we can rapidly validate an equivalent resin. Releasing and controlling the resins against specifications, and not trade names, enhances the quality of films, facilitates change management, and improves long-term assurance of supply with fast implementation of alternative resins suppliers in case of change or discontinuation of some raw materials. 
In addition, the design space that we have established for the film extrusion provides a fast and easy way to validate implementation of new extrusion equipment should a change or a capacity ramp-up be required.
Business continuity planning consists in covering unexpected events or natural disasters affecting the supply or the manufacturing capabilities. It is best achieved by ensuring that every critical process step can be run with at least two different pieces of equipment and in two different manufacturing locations. When redundant process equipment is not available, an alternative is to maintain safety stocks that cover the demand for the time period that would be necessary to build and qualify new equipment or production lines.
We are developing a back-up resin cracker and film extruders with our suppliers and partners to consolidate our business continuity plan. In the meantime, we maintain at least two years of safety stock for resins and film.
Multiple bag-making equipment and assembly lines have been installed in multiple manufacturing locations in France, Tunisia, Puerto Rico, and China to ensure our business continuity plan for final bag assemblies. We also have contracted multiple gamma-sterilization sources and suppliers worldwide to ensure the final assurance of sterility.
Sartorius Stedim Biotech has been working over the past few years on this strategy to enhance quality, assurance of supply, and change control for our single-use bags and intelligent single-use solutions made of bags, filters, tubes, connectors, sensors, process analytical tools, and hardware.
Our Flexsafe bag range is a new benchmark meeting the most stringent customer needs for safe bioprocessing.
Jean-Marc Cappia is vice-president of marketing for fluid management technologies at Sartorius Stedim Biotech, Z.I. Les Paluds, 13781 Aubagne, 33-442-845-600: [email protected]; www.sartorius-stedim.com
You May Also Like






