- Sponsored Content
- Chromatography
Establishing Effective High-Throughput Contaminant Removal with Membrane ChromatographyEstablishing Effective High-Throughput Contaminant Removal with Membrane Chromatography
Sponsored by Sartorius
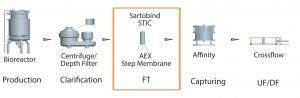
Figure 1: Placement of membrane STIC after harvest clarification step in downstream purification process (AEX = anion-exchange, FT = flow through, UF/DF = ultrafiltration/diafiltration)
Bharat Serums and Vaccines Limited (BSV) in India conducted a study based on effective removal of host cell proteins (HCPs) from a recombinant hormone with a wide isoform profile in the acidic range imparting drug-product activity. Because the hormone and HCPs have a similar range of active species, the purification process with conventional chromatography resins had difficultly removing those HCPs from the active isoforms of the hormone. To solve that issue, a membrane chromatography technique was implemented.
Our initial choice of membrane was anion exchange because we wanted to eliminate HCPs (typically negatively charged) from the bulk drug substance. One major challenge is the characteristic of higher conductivity, which usually hinders HCP clearance in flow-through mode with all the ligands for both resin- and membrane-based chromatography.
So Sartorius Stedim Biotech suggested that we try Sartobind STIC membranes in the process for effective removal of HCPs. Such membranes are a new group of salttolerant interaction chromatography (STIC) membrane absorbers. With the primary amine ligand, they bind negatively charged impurities such as DNA, HCPs, endotoxins, and viruses at much higher salt concentrations than those of conventional Q matrices. The new membranes eliminate the need for feed- stream dilution before flow-through polishing of recombinant proteins and monoclonal antibodies (MAbs).
BSV had tried conventional resins by diluting the bulk drug substance to the desired low conductivity they required, but the results obtained for HCP reduction had not been satisfactory. To overcome those challenges, we introduced STIC. To evaluate STIC in process, we designed a protocol with respect to pH, flow rates, and the introduction step. Membrane binding capacity and protein transmission were determined during initial trials to optimize flow rates and the pH range to achieve the maximum log reduction of HCPs.
Our study was intended to optimize the contaminant (HCP) retention/removal using Sartobind STIC membrane chromatography for production of a recombinant hormone product. This membrane also was preferred to overcome purification challenges presented by high conductivity (∼20 mS/cm) substances, which also would help eliminate the requirement for dilutions of the bulk substance.
A number of experiments were conducted to determine the most reliable conditions to achieve the desired level of HCPs in a bulk. First, we kept the flow rate constant irrespective of the downstream step to check the log reduction of HCPs. Those results showed that the HCP log-reduction values after the harvest clarification were better than those at the intermediate step.
We decided to continue with the process step. We varied the process modification by running the Sartobind STIC membrane chromatography at different flow rates to determine the effective residence time. For this experiment, we adjusted the flow rates to five and seven times the membrane volume to evaluate efficiency at different flow rates. Results were comparable, and we decided to go with a higher flow rate to save time at higher scales.
After selecting the flow rate and process step, we varied the pH to observe the change in membrane efficiency. We ran the membrane at two different pH values, keeping the flow rate and the conductivity constant. We observed that results at neutral pH were satisfactory and reduction was almost 97%.
In this trial/application note, we show the successful reduction of HCPs from harvested clarified bulk drug substance. The reduction is significant (≥97%) after treatment with STIC membrane. We also checked the consistency of the membrane after 10 uses, and we observed that the efficiency of the membrane was not affected by multiple cleaning cycles.
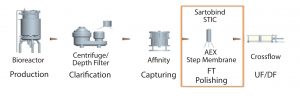
Figure 2: Placement of membrane STIC after capturing step in downstream purification proces
Determination of the Process Step
Placement of the Sartobind STIC membrane in a process is important (Figures 1 and 2). This decision should take into account the performance of the membrane at different stages. We recommend use just after harvest clarification so as to reduce the loads on further chromatography steps. It also helps to reduce carryover of contaminants along the process.
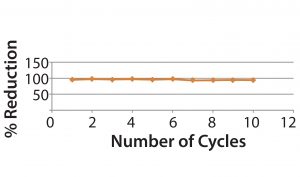
Figure 3: Host cell protein reduction values
Percentage Reduction of Host Cell Proteins
We performed initial studies with three variables: flow rate, conductivity, and pH. After conducting various experiments, we narrowed flow rate and pH ranges. After keeping those factors constant, we conducted additional trials and concluded that the range of conductivity was 15–18 mS/cm for optimal performance of the membrane for HCP removal.
Removal of Host Cell Proteins Material: We used the following materials: a Sartobind STIC PA (primary amine) Nano capsule (1 mL), an Äkta Prime chromatography system (GE Healthcare), harvested clarified culture fluid, an HCP detection kit, buffer (see “Buffers” box below), and 1 M NaOH.
Buffers and Solutions |
|---|
The following buffers were used with the membrane-chromatography method during the HCP removal process: 15 mM PO4 + 150 mM NaCl, pH 7.00, electrical conductivity (E/C) range of 15–18 mS/cm. |
The following buffers were used with the resin-based chromatography method: preload conditions were pH 7.50, E/C range was 15–18 mS/cm. For equilibration, phosphate–sodium chloride buffer, pH 7.50, E/C range of 2–mS/cm. Product elution buffer was phosphate–sodium chloride buffer, pH 7.50, E/C range 220–300 mS/cm. Cleaning solution was 0.1 M NaOH. |

Table 1: Trials performed
Methods: All parameters were predefined by performing different screening studies. It is important to remove air from the unit completely. For the Nano device, we recommend filling a 10–20 mL Luer syringe with equilibration buffer and connect to the Nano capsule.
Method procedure is as follows: Hold the capsule upright (outlet is up) and expel the air. If you still detect air in the filled unit, close the outlet, hold the syringe up, and move the plunger slightly up and down so that the air bubbles ascend into the syringe. Small air bubbles observed directly below the inlet do not disturb separations. The capsule will function normally as long as the small air bubbles remain outside of the membrane bed. Wash the membrane to remove all the storage liquid. All the given parameters should be fed in the system before the run. We adjusted the following parameters before bulk loading:
pH adjusted to pH 7.00
flow rate fixed at 7 mL/min
UV at Autozero with buffer.
Once the membrane is washed and equilibrated, the bulk is loaded onto the device with the specific flowrate and pH.
Flow-through is collected in fractions to check the membrane’s breakthrough point. That point also is used to determine the exact binding capacity of the membrane, which subsequently helps in the sizing of the membrane during scale up. Those collected fractions also are given in a quality check to determine protein concentration as well as for the HCP retention check.
Cleaning the Device: Cleaning and regeneration can be done by using 1 M NaOH. This also will help to ensure the removal of contaminants from the membrane surface. This cleaning can be carried out for 20 minutes with the same flow rates used during batch operation. After cleaning, the membrane should be washed with equilibration buffer 15 mM PO4 and 150 mM NaCl, pH 7.00, conductivity range 15–18 mS/cm. Care should be taken not to use the purified water or water for injection (WFI) to remove buffer. The device can be reused for 10 cycles to determine the consistency of the membrane performance.
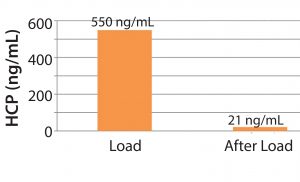
Figure 4: Average HCP reduction over 10 cycles
Results
Figures 4 shows HCP clearance with Sartobind STIC PA membrane filters. At a load condition of pH 7, 15–18 mS/cm, HCP was cleared to 21 ng/mL from HCP load of 550 ng/mL. We can conclude that the STIC can reduce HCP more than 20 times the initial HCP load compared to two times with conventional chromatography resin during downstream operations. This exemplifies the ability of the membrane chromatography method to operate at high conductivity conditions without adverse effects on HCP clearance.
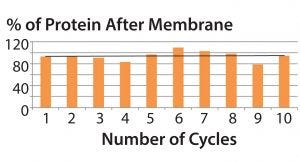
Figure 5: Average yield per 10 cycles
Efficiency of Membrane Chromatography
The Sartobind STIC PA membrane can overcome limitations of HCP removal at high conductivities because it uses a primary amine ligand that is attached to a cross-linked, regenerated macroporous cellulose base matrix. This novel combination results in higher binding capacities of impurities at high conductivities, which can resolve facility fit limitations caused by feed-stream dilution when using chromatography media that necessitates operating at low conductivities. Using STIC membranes in downstream processes also can help eliminate the problems caused by high protein concentrations during loading.
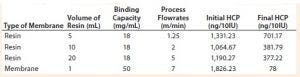
Table 2: Comparative analysis of HCP removal by membrane STIC and chromatography resins
Resin and Membrane Comparison: We conducted studies to compare membrane and resin-bead chromatography removal of HCPs. We used an R&D setting with small-scale volumes of membrane at 1 mL with operational flow rates at 7 mL/min, resin volume from 5 to 20 mL, and an operational one-fourth CV of flow rate used during membrane run. We evaluated the membrane for its advantages (low buffer consumption, less process time, high mass transfer, and low cost).
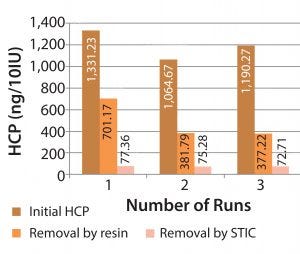
Figure 6: HCP removal by resin-based and salt-tolerant interaction membrane chromatographies
Figure 6 shows results of some comparative studies, including the volume of resin and the membrane used to remove HCPs. Final HCP reduction values of membranes are better than those of the resins. We also observed that the time required to process the same volume was reduced by 50%, and cleaning time was reduced by 60%. Using membrane chromatography also eliminates the capital expenditure cost of hardware and the work power required for the same results. Membrane chromatography is easy to use because the devices are plug-and-play and do not require evaluation (e.g., height equivalent of theoretical plates, HETP).
Additional Reading
Martin Leuthold M, et al. A New Scale-Down Membrane Adsorber Device for Process Development and Validation. BioPharm Intl. 25(12) 2012.
Scale Up with Sartobind SingleSep. Sartorius Stedim Biotech GMbH. Application Note SL-4042-e07081.
Glynn J, et al. Downstream Procecessing 2010. BioPharm Intl. 22, 2009: s16–s20.
Janice Lim, et al. Technical and Economic Benefits of Membrane Chromatography During Polishing Steps. BioPharm Intl. 23(8) 2010.
Pranav Gupte is a senior research executive at Bharat Serums and Vaccines Ltd., 3rd Floor Liberty Tower, Plot No. K-10, Kalwa Industrial Estate, Airoli, Navi Mumbai, India 400 708; [email protected]. Mahesh Gavasane is deputy general manager (Mahesh. [email protected]); Anita Samagod, PhD, is assistant vice president ([email protected]); Gautam V. Daftary, PhD, is managing director ([email protected]). Mihir Patel is a platform specialist, downstream, at Sartorius Stedim India Pvt. Ltd.
You May Also Like






