- Sponsored Content
Establishing Microbiological and Particulate Controls When Reworking a Sterile Drug ProductEstablishing Microbiological and Particulate Controls When Reworking a Sterile Drug Product
September 19, 2023

Reworking of finished sterile-product batches requires particular attention to maintaining microbial and particulate control. The strategy is permitted with regulatory agreement and observation of regulations such as 21 CFR 820 (1). Note that reworking is distinct from reprocessing. Under ICH Q7, reprocessing is confined to intermediates, whereas reworking is reserved for finished products (2). Reworking includes steps that were not part of an initial biomanufacturing process. The reworked steps are process elements that are designed either to bring an out-of-specification product into specification or to return a process that has shifted outside its validated operating range to an acceptable state of quality.
For reworking, a pharmaceutical manufacturer needs to have in place a procedure that includes details on retesting and reevaluation criteria for the nonconforming product after rework. Reworking should be associated with a deviation record (for the original reason to consider rework) and executed under change control. As well as standard product-release tests, additional tests will be required during the rework activity, and the product will need to undergo a stability study.
The reworking of a drug product should be an exceptional activity, and repeated occurrences suggest a lack of operational robustness and potential process-validation concerns. A rework should be attempted only after a product’s critical quality attributes (CQAs) have been identified and a design space has been optimized. Manufacturers must have a mature understanding of the impact of factors, including process parameters, that can influence product quality.
Designing and undertaking a rework require two aspects that are central to ensuring that a robust sterility-assurance system is in place: a contamination-control strategy and a quality risk management (QRM) approach. Those frameworks should provide a structural basis for assessing contamination potential (3).
Based on the principle that sterile products thought to be contaminated are not reworked (not least because of the near impossibility of removing microbial toxins), the primary risk that arises during rework is from extrinsic microbial hazards. Perhaps two of the most important considerations are the risk for microbial contamination introduced from the rework activity itself and the time taken to complete the activity and filter the product (the hold period). In particular, if a final product will support microbial growth under the conditions of the holding period, then that time should be limited to ensure that microbial growth does not exceed filtration efficiency; to prevent microbial build-up on a filter; and to control the generation of microbe-derived toxins, of which endotoxin typically raises the greatest concern. Measuring endotoxin levels provides information about risk involving specific types of microorganisms (Gram-negative bacteria) and about the presence of pyrogenic toxins that would be difficult to eliminate (if it is possible at all) (4).
Below, I present a case study of a rework process, including an outline of process controls, a review of environmental controls, a statement of intent for environmental and bioburden monitoring, and a review of the sterility-assurance impact.
Preliminary Considerations
This case study focuses on a sterile, liquid, proteinaceous drug product for parenteral administration. The product’s license specified that bulk drug product be processed through a final sterilizing-grade filter within 24 hours. That condition necessitated time-based parameters of
a maximum decapping time of
12 hoursan additional hold time of 12 hours before filtration (to allow for formulation adjustments, e.g.)
filtration at times listed in the product license.
The rework process occurred within an EU good manufacturing practice (GMP) grade C/ISO 14644 class 8 (in operation) cleanroom under localized unidirectional airflow (UDAF) protection. That strategy was based on the simple but accurate premise that open processing presents a higher contamination risk than does closed processing, necessitating adoption of risk-mitigation measures. A barrier device supplying clean air was an important design element of the cleanroom contamination-control system (5). The process was manual, with operators decapping vials and pouring their contents into a sterilized vessel to create the bulk for sterile filtration, within an allocated time period (6).
If microorganisms are deposited into a product during reworking, then the possible outcomes are microbial survival without growth, survival with growth, or death. Those outcomes differ by product, process, time, and temperature. They also depend on the type of microorganisms deposited, their physiology, and their oxygen requirements in the receptacle headspace. Given that most cleanroom contamination — including that from a manual rework process — originates primarily from employees, mesophilic aerobic bacteria (and fungi) are the most probable challenges; therefore, such organisms pose the highest risk (7). Thus, processes occurring at “room temperature” present a greater theoretical risk than do processes performed at more extreme temperatures. In this case study, such a hazard was present because the product (proteinaceous) could support microbial growth and maintain contaminating organisms in a physiological state that encouraged their generation, hence leading to a potential rise in bioburden over time (8). To improve detection, the room was subject to environmental monitoring during reworking activity.
The Rework Process
Product recovery and process reworking followed the general workflow described below. Under UDAF, personnel removed each bottle’s overseal and stopper, then poured the contents through an autoclaved sieve into an autoclaved stainless-steel pooling vessel. In general, whether to use vertical or horizontal UDAF depends on the workspace design. The objective is to supply clean air in a way that forces particles in a uniform direction, from the cleanest area (under the filter face) to the exit area (9). Note that air flow is subject to disruption when it reaches an object, including, for instance, an operator. Wakes, turbulence, and vortices form thereby, becoming areas of potential contamination deposition. The length of a wake is about twice the size of the object (10).
Vessels underwent process controls relating to equipment cleaning and hold times (both precleaning and postcleaning) and sterilization. Pooled material was held in a small vessel and then transferred into a suitably sized, tared process vessel through an enclosed transfer pipe system by peristaltic pump. As required by the product license, operators completed rebulking within 12 hours of the first vial being opened. Note that because the number of bottles being reworked is typically <20,000, the process should take significantly less time. Time can be controlled through procedure.
After completing the rebulking process, operators weighed the recovered material and recorded needed values in associated documentation. Material was mixed in the bulk pooling vessel for no less than 15 minutes. After mixing and sampling (see below for information about required testing, including that for microbial bioburden), the batch was transferred into an aseptic processing area through a prefilter and 0.22-µm bioburden-reduction filter using a standard procedure. Note again that this process must occur within 12 hours of completing the bulking operation. The filter must be located as close as possible to the point of filling.
New batch numbers were assigned, and filling and inspection began, following established procedures.
Hazard Identification
As per ICH Q9 (R1), risk management consists of risk assessment, control, communication, and review (11). The risk-assessment component requires identification of hazards and evaluation of the risks associated with exposure to those hazards (12). Hazard identification requires an initial problem statement. In this case study, a suitable statement was “What are the microbiological and particulate hazards involved in product reprocessing, and what measures can be taken to prevent those hazards?” Hazards relating to such assessment include
an increase in bioburden
an increase of bacterial endotoxin,
as a consequence of a bioburden increase
an increase in particulates (13).
A few factors can influence those hazards, including contamination travelling in the air stream (both microbe-carrying particles and nonviable/inert particulates). Microbe-carrying particles can deposit microorganisms directly into a product or in containers designed to hold it. The largest source of such contaminants is skin detritus. Nonviable particles include material fibers and other such impurities that can adulterate a product. Contamination can come directly from personnel, such as through touching of surfaces and components. Excessive length of activity also can increase low microbial counts to high-level bioburden challenges: Taking more time gives microbes an opportunity for exponential binary fission sufficient to transition a population from its lag phase to its exponential-growth phase. Such a risk would be enhanced if the initial contaminant is a Gram-negative bacterium that is adapted to nutrient-depleted conditions and other external stresses.
Although such factors would result in severe contamination transference, application of a sterilizing-grade filter coupled with finished-product testing (for endotoxin and sterility) mitigates both the risk of contaminated product entering the aseptic processing area and of a contaminated product being released. But a concern lies in the strategy of processing contaminated batches only to reject them at a later date. Doing so uses a filling slot for a batch that ultimately will be rejected when such a slot could have been used for a product that could be released to address a patient need.
Table 1 summarizes factors related to contamination likelihood that were reviewed in the case study. For the hazards identified, likelihood also depended on the degree of control. Controls can be divided into different categories, as outlined below.
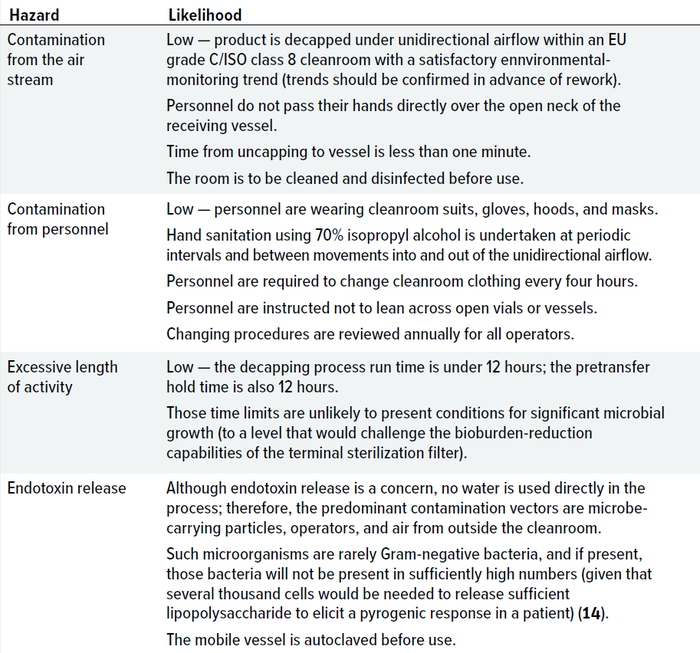
Table 1: Likelihood of contamination from hazards identified during the case study.
Process Controls: The manufacturer reviewed process controls to help minimize risks. Controls were deemed sufficient based on several considerations. For instance, the reworking process followed a standard operating procedure (SOP), with activities taking place under downward UDAF and being performed in compliance with current good manufacturing practice (CGMP) requirements.
Bottles to be reworked were transferred from an inspection, labelling, and packaging (ILP) area through a transfer airlock from an EU grade D area to a grade C cleanroom. The staged and controlled process was designed to minimize contamination transfer from ILP to cleanrooms. To protect the bottles’ integrity, operators removed them from their storage cages and placed them in security boxes for transport into the process area. Each box was labelled with a batch number and vial quantity, providing traceability and preventing mix-ups, as required in ICH Q7, section 8.17 (2). Except during transfer, bottles were not left or worked on in any area that lacked some form of environmental monitoring. That enabled verification of the background environment. In the process area, operators performed a reconciliation of the supplied bottles, including checks of batch numbers and container integrity. That measure also helped to provide traceability and prevent mix-ups, as required in ICH Q7 (2).
Operators performed rework activities in an EU grade C (ISO class 8) cleanroom, with air filtered into the room using H14 class high-efficiency particulate-absorbing (HEPA) filters
(EN 1822) and a pressure differential maintained between the room and access corridor. That strategy ensured a high level of background environmental control. All equipment used for product-contact activities was cleaned based on a verified procedure and decontaminated using steam sterilization by autoclaving. Vessels also were verified as dry before use to minimize starting bioburden. All open-rework pooling activity — e.g., decapping and pooling of bottles into the smaller pooling vessel — was performed under UDAF to mitigate airborne contamination transfer. Personnel were gowned, and they wore gloves, masks, and headcovers to minimize human-derived contamination. They also practiced hand sanitization.
Operators leveraged an enclosed system to transfer product into the bulk pooling vessel held in the same room as the smaller container. Use of an enclosed system prevents contamination ingress. Bulk product was transferred into a sterile vessel inside the aseptic filling suite through a sterilizing-grade filter. Doing so is an established process designed to produce a sterile filtrate. It is important that filter validation has been performed using a representative product (or justified placebo). Validation must have accounted for total filtration time and demonstrated suitable bacterial retention.
Environmental controls supported the above assessments. The bracketing control was the performance of reworking activity within a cleanroom. It is important for reworking of sterile products to occur in a process-area cleanroom. For this case study, the room needed to be EU GMP grade C, which is equivalent to ISO 14644 class 8 in operation and class 7 at rest. The room was selected based on review of 12 months of environmental-monitoring data. It exhibited a satisfactory trend in microbiological control and a good record in relation to excursions above action levels. The cleanroom also was verified as meeting the required particle-classification standard. It was assessed for particulates and viable-microbe counts as part of a weekly environmental monitoring program (although, for the rework exercise, monitoring was conducted as the activity took place). Before use, the room was cleaned and disinfected. A facility monitoring system was in place to ensure that the cleanroom was within the desired temperature range (18–25 °C) during the process.
To confirm compliance with the required cleanroom classification, the room was monitored continuously for pressure cascades to neighboring areas. During rework activity, downward UDAF at a velocity of 0.45 m/s (±25%) provided additional product protection.
Personnel Controls: Personnel working in the cleanroom wore full cleanroom suits with dedicated footwear, head covers, masks, and gloves. Operators’ gloves were sanitized at intervals of not less than 15 minutes using 70% isopropyl alcohol, with the disinfectant applied for 30 seconds. Each staff member was trained in good cleanroom practices.

Detection Methods
After hazards are identified and control measures are considered, risks of hazards can be evaluated and qualified. When hazards remain at a level that holds a reasonable likelihood of contamination transfer, it is important to have in place a detection system to alert the facility. Although much conventional microbiological analysis involves postevent assessment (because of the necessity of growing microorganisms on culture media), implementation of a detection process still enables assessment of product-contamination risks. Table 2 highlights specific detection methods.
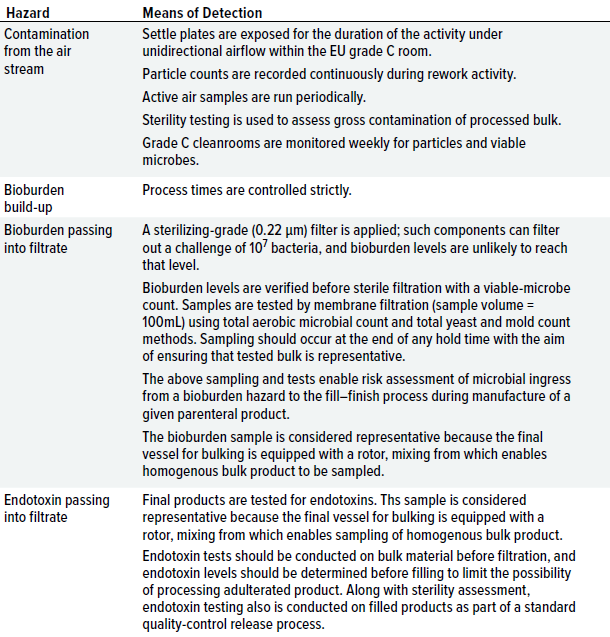
Table 2: Measures for detecting contamination hazards identified during the case study.
Sampling technique is important to prevent “false positives” that can arise from cross-contamination. When establishing an initial process, a manufacturer should have determined whether samples could be taken aseptically into a sterile container; however, a reworked process might leverage different equipment, such as a new bulk-containing vessel. Hence, a manufacturer must review such considerations again before beginning rework to determine how bioburden and endotoxin samples can be taken aseptically. Disposable systems that are closed to the environment (e.g., bioprocess bags) can help to prevent adventitious contamination from occurring through sampling.
This case study involved conventional methods for microbiological analysis, including growth-dependent bioburden testing. A higher level of assurance can be built into bioburden assessment using faster, growth-independent methods such as those based on extrinsic fluorescence. Similarly, environmental-monitoring methods based on intrinsic fluorescence can yield more-direct information about the state of environmental and personnel control during a rework process.
This case study involved some direct testing of bioburden and bacterial endotoxin. Although a starting product is probably sterile, rework activity can introduce risks for microbial ingress into bulk material. Such risks are likely to increase over time given the manual nature of the operation. To ensure bioburden control, a sample should be taken from the homogenous bulk just before prefiltration (0.22-µm filter). Once bulk material has been transferred into the aseptic processing area, another sample should be taken just before final sterilizing filtration. Samples for bioburden testing should have a volume of >100 mL; analysis should reveal no more than 10 colony-forming units (CFU)/100 mL of sample. For endotoxin testing, samples should be 10–20 mL in volume and contain no more than 1 endotoxin unit (EU)/mL of sample.
In this case study, the reworked product was filled aseptically in the aseptic-processing room under an EU GMP grade A/ISO 14644 class 5 environment according to existing procedures. Filling occurred under continuous environmental monitoring, and personnel performed a full array of finished-product testing, including sterility testing, bacterial endotoxin testing using a Limulus amebocyte lysate (LAL) methodology, and analysis for particles and impurities.
Environmental Monitoring: Environmental monitoring supported the process detection methods discussed above. Assessment of the cleanroom setup and unidirectional-airflow device provided indirect data about the operating environment because detection of contamination in the background environment does not necessarily entail the transfer of contamination into a product.
Before rework activity, the UDAF device was assessed for airborne particulates. Doing so demonstrated EU GMP grade A/ISO 14644 class 5 air supply. If particulate counts had been higher than allowable ranges, then rework would not have started, and remediation actions would have been taken.
During rework activity, a viable-microbiological monitoring program was executed for the cleanroom. The program took place underneath a UDAF device. Resulting data yielded information about the quality of the cleanroom environment. Any excursions from action levels were considered through impact assessment. Data from such programs are used primarily to assist with root-cause analyses when high bioburden levels are detected in bulk product; they are not used to infer product-contamination causality directly.
Discussion
Herein, I have used a case study to explore the rework process for a sterile drug product, focusing on three product quality attributes: sterility, endotoxin, and bioburden. I have considered risks to the point of sterile filtration. That is obviously not the end of microbial risks that can compromise a drug product; thus, effective aseptic processing using barrier technology must be maintained (15). In outlining the rework process, I have identified different hazards, describing them according to their severity and likelihood.
After considering such factors, manufacturers can evaluate risk reduction and mitigation measures, and once risks pertaining to different hazards have been reduced to their lowest possible levels, monitoring and other forms of detection can be explored. All of these analyses need to be captured in a risk assessment. To ensure that the resulting document remains suitable, a manufacturer should review it regularly and evaluate the change-control process, during which changes with probable process impacts are proposed.
The main requirements for reworking of a sterile liquid product are that
the process is followed
controls are in place
the recovery process is described
environmental controls are established, including UDAF protection and appropriate staff training
environmental monitoring is performed, including preactivity UDAF assessment and monitoring during batch processing
bioburden is controlled, with samples taken at time-based intervals and before final-filtration bulk assessment
sterility is assured.
When each of the above factors is classed as satisfactory, then generally, the severity and likelihood of microbial contamination will be low.
References
1 21 CFR 820. Quality System Regulation, Subpart I: Nonconforming Product. US Food and Drug Administration: Silver Spring, MD, 7 June 2023; https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=820&showFR=1&subpartNode=21:8.0.1.1.12.9.
2 ICH Q7. Good Manufacturing Practice for Active Pharmaceutical Ingredients. European Medicines Agency: Amsterdam, Netherlands, November 2000; https://www.ema.europa.eu/en/documents/scientific-guideline/ich-q-7-good-manufacturing-practice-active-pharmaceutical-ingredients-step-5_en.pdf.
3 Sandle T. Establishing a Contamination Control Strategy for Aseptic Processing. Amer. Pharm. Rev. 20(3) 2017: 22–28; https://www.americanpharmaceuticalreview.com/Featured-Articles/335458-Establishing-a-Contamination-Control-Strategy-for-Aseptic-Processing.
4 Sandle T. Assessing Process Hold Times for Microbial Risks: Bioburden and Endotoxin. J. GXP Compl. 19(3) 2015: 1–9; https://www.researchgate.net/publication/282661901_Assessing_Process_Hold_Times_for_Microbial_Risks_Bioburden_and_Endotoxin.
5 Whyte W. A Cleanroom Contamination Control System. Eur. J. Parenter. Pharm. Sci. 7(2) 2022: 55–61.
6 EMA/CHMP/CVMP/QWP/BWP/850374/ 2015. Guideline on the Sterilisation of the Medicinal Product, Active Substance, Excipient and Primary Container. European Medicines Agency: Amsterdam, Netherlands, 2016; https://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2016/04/WC500204724.pdf.
7 Clontz L. Microbial Limit and Bioburden Tests: Validation Approaches and Global Requirements. CRC Press: Boca Raton, FL, 2013; https://www.routledge.com/Microbial-Limit-and-Bioburden-Tests-Validation-Approaches-and-Global-RequirementsSecond/Clontz/p/book/9781420053487.
8 Cundell T. GMP Challenges with Time Limitations in Pharma Manufacturing. PDA Lett. 8 September 2022; http://www.pda.org/pda-letter-portal/home/full-article/gmp-challenges-with-time-limitations-in-pharma-manufacturing.
9 Whyte W, Agricola K, Derks M. Airborne Particle Deposition in Cleanrooms: Relationship Between Deposition Rate and Airborne Concentration. Clean Air Contain. Rev. 25(1) 2016: 4–10; https://www.researchgate.net/publication/311425193_Airborne_particle_deposition_in_cleanrooms_Relationship_between_deposition_rate_and_airborne_concentration.
10 Hoffmann WC. A Study of the Influence of Variables in Air Velocity Measurement in a Unidirectional Air Flow Device or Environment. Eur. J. Parenter. Pharm. Sci. 18(4) 2013: 118–123; https://www.researchgate.net/publication/286058054_A_study_of_the_influence_of_variables_in_air_velocity_measurement_in_a_unidirectional_air_flow_device_or_environment.
11 ICH Q9(R1). Quality Risk Management. ICH: Geneva, Switzerland, January 2023: https://database.ich.org/sites/default/files/ICH_Q9%28R1%29_Guideline_Step4_2022_
1219.pdf.
12 Tidswell EC. Risk Profiling Pharmaceutical Manufacturing Processes. Eur. J. Parenter. Pharm. Sci. 9(2) 2004: 49–55.
13 Tidswell EC. Bioburden Control. Aseptic and Sterile Processing: Control, Compliance, and Future Trends. Sandle T, Tidswell EC, Eds. DHI Publishing: River Grove, IL, 2017: 163–192; https://www.pda.org/bookstore/product-detail/4256-aseptic-and-sterile-processing.
14 Tidswell EC, Agalloco J, Tirumalai R. Sterility Assurance: Current and Future State. PDA J. Pharm. Sci. Technol. 76(3) 2020: 012526; https://doi.org/10.5731/pdajpst.2020.012526.
15 Zhang G, Meredith TC, Kahne D. On the Essentiality of Lipopolysaccharide to Gram-Negative Bacteria. Curr. Opin. Microbiol. 16(6) 2013: 779–785; https://doi.org/10.1016/j.mib.2013.09.007.
16 Agalloco JP. Increasing Patient Safety By Closing the Sterile Production Gap: Part 1, Introduction. PDA J Pharm. Sci. Tech. 71(4) 2017: 261–268; https://doi.org/10.5731/pdajpst.2016.007104.
Resources from the Author
Author Tim Sandle curates Microbiology News, a weekly LinkedIn newsletter that features engaging discussions about key topics in microbiological detection and assessment for biopharmaceutical manufacturing and beyond. A few recent articles are listed below. Visit LinkedIn for the complete newsletter archive.
Tougher Than All the Rest? Why Micrococcus luteus Can Survive for a Long Time in Your Cleanrooms. 5 August 2023; https://www.linkedin.com/pulse/tougher-than-all-rest-why-micrococcus-luteus-can-long-dr-tim.
Go with the Flow: Why the Reynolds Number Is Important for Microbial Control. 22 July 2023; https://www.linkedin.com/pulse/go-flow-why-reynolds-number-important-microbial-dr-tim.
Containers, Closures, and Measurements To Support the Assurance of Sterility. 8 July 2023; https://www.linkedin.com/pulse/containers-closures-measurements-support-he-assurance-dr-tim.
Resources From the BPI Archive
Kouda R, Tabassum S, Dolan D. Degradation of Biopharmaceuticals During Cleaning Processes: Comparing Two Different Analytical Methods for Assessment with Bispecific Antibodies. BioProcess Int. 21(6) 2023: 28–38: https://bioprocessintl.com/manufacturing/facility-design-engineering/degradation-of-biopharmaceuticals-during-cleaning-processes-comparing-two-different-analytical-methods-for-assessment-with-bispecific-antibodies.
Sharnez R, et al. Cleaning Validation Acceptance Limits for Biological Process Residues: Part 1 — Acceptable Exposure of Degraded Proteins Based on Reference Immunogens. BioProcess Int. 21(5) 2023: 26–31, 43; https://bioprocessintl.com/manufacturing/validation/cleaning-validation-acceptance-limits-for-biological-process-residues-part-1-acceptable-exposure-of-degraded-proteins-based-on-reference-immunogens.
Nieuwenhuizen P. Focus on Aseptic Processing: A Report from the September 2022 PDA Annex 1 Event in Amsterdam. BioProcess Int. 21(3 suppl.) 2023: 3–6, 16; https://bioprocessintl.com/business/regulatory-affairs/focus-on-aseptic-processing-a-report-from-the-september-2022-pda-annex-1-event-in-amsterdam.
Boulanger R, et al. Spontaneous Infection: Did You Leave the Back Door Open to Your Cultivation Suite? BioProcess Int. 19(9) 2021: 36–43; https://bioprocessintl.com/upstream-processing/biochemicals-raw-materials/viral-contamination-did-you-leave-the-back-door-open-to-your-cultivation-suite.
Kamstrup S. How Much Harm Can a Single Droplet Do? Considerations for a Viral Inactivation Step. BioProcess Int. 18(10) 2020: 46–52; https://bioprocessintl.com/downstream-processing/viral-clearance/how-much-harm-can-a-single-droplet-do-considerations-for-a-viral-inactivation-step.
BPI editorial advisor Tim Sandle, PhD, CBiol, FIScT, is head of QA and GxP compliance at Bio Products Laboratory (part of Kedrion) in Elstree, UK, and a visiting tutor at University College London and the University of Manchester; [email protected].
You May Also Like






