- Sponsored Content
Improving Product Quality By Lowering Glucose ConcentrationImproving Product Quality By Lowering Glucose Concentration
August 15, 2014
 Cell culture is widely used for stable expression of monoclonal antibodies with posttranslational modifications. Despite having a stable cell line, a protein product is still in danger of improper modifications from chemical interactions with media. For example, the primary amino groups on a protein are susceptible to reactions with reducing sugar molecules. In the case of a reaction with glucose, this is called
Cell culture is widely used for stable expression of monoclonal antibodies with posttranslational modifications. Despite having a stable cell line, a protein product is still in danger of improper modifications from chemical interactions with media. For example, the primary amino groups on a protein are susceptible to reactions with reducing sugar molecules. In the case of a reaction with glucose, this is called
glycation. The relationship between glucose concentration and glycation is explored here.
Methods:
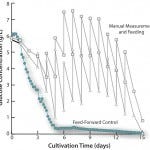
FIGURE 1: Glucose measurements from three fed-batch runs. Run 1 (square) and run 2 (triangle) were fed once daily. Run 3 (circle) was fed continuously
Chinese hamster ovary (CHO) cells were grown in fed-batch mode in a 2-L glass bioreactor. Offline glucose measurements were obtained using a YSI 7100 system with glucose membrane and two-point calibration. Online measurements were obtained with Biosenz analyzer, with glucose sensor and automated four-point calibration. Glycation of proteins was determined using ESI-TOF analysis.
Results:
Two runs proceeded with daily bolus feed of glucose. Manual samples before and after the feed were analyzed for glucose concentration. In run 1, 17% glycation was obtained. In run 2, where the glucose concentration was lower than run 1, glycation was 10%. To further explore the effect of keeping the glucose concentration low, run 3 was performed with feed-forward glucose additions and automated online monitoring of the glucose concentration. Using this strategy, glucose was maintained <1g/L. By preventing the higher glucose concentration that resulted from bolus feeding, glycation was eliminated in run 3.

Biosenz instrument with sample filter probe connected in 1-L jacketed vessel
Conclusions:
Automated measurement of glucose at frequent intervals using the Biosenz analyzer enables safe operation at lowered glucose concentrations. When glucose was maintained <1g/L, glycation was eliminated without decreasing productivity or yield.
Data were obtained courtesy of Genmab (Utrecht, NL).
Reference
1. Yuk, IH. Controlling Glycation of Recombinant Antibody in Fed-Batch Cell Culture. Biotechnol. Bioeng. 2011: 108(11) 2600-2610.
Ann D’Ambruoso
is product manager, small-scale technologies, 1180 Chess Dr Foster City, CA; 1-650-578-1396;
[email protected]; www.applikonbio.com.
You May Also Like






