- Sponsored Content
Integrated and Scalable Continuous Chromatography: The Cadence BioSMB PD System from PallIntegrated and Scalable Continuous Chromatography: The Cadence BioSMB PD System from Pall
Sponsored by Cytiva
 Bioprocess intensification leads to more efficient, flexible, and cost-effective biopharmaceutical manufacturing facilities. Single-use systems designed for continuous operation facilitate process intensification.
Bioprocess intensification leads to more efficient, flexible, and cost-effective biopharmaceutical manufacturing facilities. Single-use systems designed for continuous operation facilitate process intensification.
Pall Life Science’s Cadence™ BioSMB PD platform is the first disposable flow path, continuous multicolumn chromatography solution that is fully scalable from a process development laboratory to good manufacturing practice (GMP) manufacturing. It provides an economically viable, disposable chromatographic solution for even the most demanding and costly chromatographic steps.
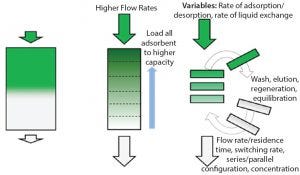
Figure 1: Principles of simulated-moving bed technology
During multicolumn countercurrent (also known as simulated moving bed, or SMB) chromatography, all chromatographic steps normally run in a batch mode are conducted simultaneously in different “zones” (Figure 1). Chromatographic media reaches full lifetime within a campaign through rapid cycling, making disposable use of even the most expensive chromatographic media possible.
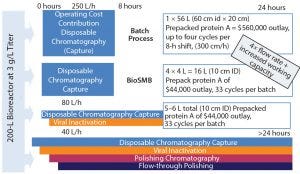
Figure 2: Significant savings can be achieved with disposable chromatography (Cadence BioSMB)
Typically, in a continuous multicolumn chromatography process, at least 40% more protein per liter of chromatography media is bound than for a batch process. Specific productivity also is significantly higher, allowing the same process performance with 50–85% less chromatography media. The savings for purification of a 3-g/L feed from a 2,000-L bioreactor using the Cadence BioSMB PD system can be quite significant (Figure 2).
BioSMB also allows for significant savings in buffer consumption. Its smaller operational footprint can lead to decreased tank sizes, facility utility requirements, and water for injection (WFI) usage, and a disposable flow path eliminates the need for packing skids and large stainless steel columns, as well as their supporting infrastructure. Overall, the use of fewer consumables results in supply chain, warehousing, and risk management advantages, and it eliminates the need for internal column packing resources.
Bringing the Advantages of Continuous Chromatography to Biopharma Processing
The specially designed and patented, integrated single-use valve cassette in the Cadence BioSMB system contains all valves necessary to fully automate a wide range of chromatographic processes, including bind–elute (protein A affinity and ion exchange), hydrophobic-interaction, size-exclusion, and multimembrane capture chromatography.
The Cadence BioSMB PD system is specifically designed to allow for easy conversion of existing batch processes into continuous chromatography steps without changing chromatographic sorbents, buffer systems, or product quality assays. Easy integration of adjacent unit operations around the BioSMB unit enables process development scientists to easily explore the added value of process integration.
A process-scale version of the Cadence BioSMB PD system that is based on the same design and can purify the output from a 2,000-L single-use bioreactor in less than eight hours will be introduced in Q1 of 2017.
Lynne Frick is director of sales, continuous bioprocessing, Marc Bisschops, PhD, is principal scientist, continuous bioprocessing, and Peter Levison, PhD, is senior marketing director, downstream processing at Pall Life Sciences; [email protected]; www.pall.com/contact; www.pall.com/main/biopharmaceuticals/product.page?id=immfd7vl.
You May Also Like






