- Sponsored Content
International Differences in Quality and Regulatory Requirements for Cellular Starting MaterialInternational Differences in Quality and Regulatory Requirements for Cellular Starting Material
August 17, 2023
https//STOCK.ADOBE.COM
Cell and gene therapy (CGT) applications have increased rapidly over the past few years, with hundreds of candidate treatments entering the clinical pipeline annually. Although North America, Asia, and Europe are driving industry growth, drug sponsors in those areas will not want to limit product distribution to their respective regions; rather, they will want to maximize patient impact. Thus, companies must understand global regulatory differences and their implications for therapy production. However, advancements in the CGT industry often outpace adjustments to regulatory guidances.
When considering supply chains for allogeneic cell therapies, in particular, we find common obstacles to treating global or even regional patient populations. Some difficulties concern how to obtain healthy, adult-donor cellular starting material, including
• differences in donor eligibility requirements among countries
• the influence of donor welfare and compensation on global distribution of resulting products
• differences in collection requirements for allogeneic starting material
• the need in some regions to apply good manufacturing practices (GMPs) to starting-material procurement.
Cell-Therapy Regulation
In the United States, cellular therapies are regulated by the Food and Drug Administration (FDA), primarily by its Office of Therapeutic Products (OTP). The most critical regulation that is unique to the cell-therapy space is 21 Code of Federal Regulations (CFR) Part 1271 on “Human Cells, Tissues, and Cellular and Tissue-Based Products,” which focuses on preventing introduction and transmission of communicable diseases from donors to patients (1).
I have found two other guidances to be particularly useful when navigating requirements for cell-therapy starting material. The 2007 “Eligibility Determination for Donors of Human Cells, Tissues, and Cellular and Tissue-Based Products” provides additional detail and context regarding donor eligibility (2). Note that the FDA Center for Biologics Evaluation and Research (CBER) plans to release a draft guidance late in 2023 that ultimately will amend the 2007 guidance (3). The second key regulation is the 2020 guidance on “Chemistry, Manufacturing, and Control (CMC) Information for Human Gene Therapy Investigational New Drug Applications,” which contains additional information about starting-material requirements (4).
Health Canada regulates biologics and transplant products in Canada. The agency has two primary sets of regulations that foreign drug developers must understand to work within the country. The first set includes biologics regulations that apply to products undergoing manipulation to create a cell or gene therapy and include information on activities such as testing and donor clearance (5). The second set concerns regulations for cells, tissues, and organs (CTO) (6).
In the United Kingdom, cell-therapy starting material can be collected by blood or tissue establishments. The latter organizations are regulated by the Human Tissue Authority (HTA) under the Human Tissue (Quality and Safety for Human Application) Regulations of 2007. Blood centers are regulated by the Medicines and Healthcare Products Regulatory Agency (MHRA) under the Blood Safety and Quality Regulations.
Generally, the European Union regulates cellular starting material under Directive 2002/98/EC on safety and quality of human blood and blood components or Directive 2004/23/EC on safety and quality of human tissues and cells and their implementing acts. Those guidelines have been in place for about 20 years. Although they define boundaries for starting material, they also leave a good amount of interpretation to individual member states.
In July 2022, the European Commission adopted a proposal for a new regulation on blood, tissues, and cells (BTC) (7). The new guidance would repeal and replace current blood and tissue directives with the goal of increasing harmonization with other global statutes. After consultation, agreement, and adoption, the finalized regulation would come into force with a transition period of two to three years, depending on particular provisions.
Donor Eligibility
In general, starting material for allogeneic cell therapies must be collected from donors who have met relevant eligibility requirements. Such measures are designed to protect patients from transmissible diseases and/or disease agents. Figure 1 compares starting-material requirements across regions from a US perspective. Green dots indicate a region’s full alignment with US regulations, whereas blue dots indicate differences. Cell-therapy companies must understand such nuances and account for them early in process development to prevent later disruptions to product approval and distribution.
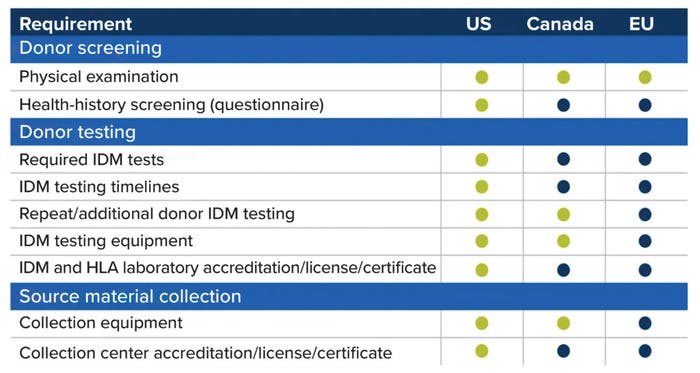
Figure 1: Comparing regulations for cellular starting materials in different regions, from a
US perspective; green dots indicate full alignment with US requirements, and blue dots
indicate differences. (IDM = infectious disease marker, HLA = human leukocyte antigen).
Eligibility requirements generally fall into two categories: donor screening and donor testing. Donor screening includes physical examination, administration of a health-history questionnaire, and review of relevant medical records. Physical-examination requirements tend to be similar across regions, but the contents of health-history assessments can differ significantly depending on a region’s specific concerns and disease-transmission risks.
Donor Testing: In the United States, donors must be tested for infectious disease markers (IDMs) as outlined in 21 CFR 1271 (1), including for hepatitis B and C, human immunodeficiency virus (HIV), syphilis, West Nile virus, and cytomegalovirus (CMV). In Europe, depending on the member concerned, additional testing may be required, such as for hepatitis A and E, parvovirus B19, and Toxoplasma gondii.
Potential donors in the United Kingdom must undergo IDM testing for HIV, hepatitis B and C, and syphilis. Good-practice (GxP) requirements from the United Kingdom Government Advisory Committee on the Safety of Blood, Tissues, and Organs also call for allogeneic donors to receive standard testing for hepatitis E, T. gondii, and CMV, as well as nucleic-acid–based screening for hepatitis B and C and HIV.
Testing timelines can differ across regions. In the United States, the standard window for taking a donor sample for IDM testing is 15 days: from seven days before to seven days after collection. Canada requires that samples be taken up to 30 days before collection of starting material. And in the European Union, samples typically are taken on the day of starting-material collection but must be taken within seven days thereafter.
Requirements also differ for testing kits and laboratories. In the United States, screening kits licensed, approved, or cleared by the FDA must be used according to manufacturer instructions in laboratories that are certified under the Clinical Laboratory Improvement Amendments (CLIA) or that have met equivalent requirements as determined by the Centers for Medicare and Medicaid Services (CMS). In the European Union, testing should be performed (when possible) using kits that have received Conformité Européenne (CE) marking. Laboratories might require special certifications. UK health authorities have released guidance about kits with CE and now UK Conformity Assessed (UKCA) marks, but the primary concern continues to be that test methods are validated for use.
Collection Centers
US facilities collecting starting material under contract with a registered establishment are exempt from registration, as listed under 21 CFR 207.13(c) (8). If a facility performs more than starting-material collection (e.g., processing), then it must register and list with the FDA — but only after the products with which it works have received market authorization. Registration is not mandatory if a cell therapy is still under an investigational new drug (IND) application.
EU facilities must be authorized to collect starting material either by their respective national or regional authorities or by internationally recognized bodies such as the Joint Accreditation Committee of the International Society for Cell and Gene Therapy–Europe and the European Society for Blood and Marrow Transplantation (JACIE) (9). Often, EU regulators take a joint approach such that collection facilities have both national authorization and JACIE accreditation.
UK tissue establishments must register with and obtain a license from the HTA for both for allogeneic and autologous processes. Blood establishments follow a similar path through the MHRA.
In Canada, all facilities for collection of allogeneic cells, tissues, and organs must be registered with Health Canada and obtain a license. If a facility collects only autologous material, then it does not require licensure.
Donor Welfare and Compensation
Donor welfare and compensation are complex topics that drug sponsors must consider carefully if they plan to distribute their therapies internationally. Compensation refers to paying donors for their ability and willingness to donate cells. Reimbursement makes the collection process accessible for donors by paying for activities such as travel to the collection site.
Historically, the European Union has held that, from a moral standpoint, donors should not be compensated. However, this topic is under discussion, particularly as it relates to the EU plasma deficit. The traditional stance could be updated given current shortages, and policy changes for plasma products might apply to cellular starting materials as well. Some member states offer financial benefits to donors — e.g., tax deductions or even flat-fee compensation. Drug sponsors and regulators hope that a revised EU policy will help to harmonize the currently fragmented approach across member states.
Canada historically has prohibited donor compensation. In the United States, however, donor compensation is acceptable for most cellular starting materials. Such an approach has some advantages. It can provide for a larger selection of diverse donors, improve donor follow-through, and increase donor retention. But sponsors also have reasons for not compensating donors. Working with material from uncompensated donors enables sponsors to distribute their therapies to a larger number of countries internationally than would be allowable with products derived from compensated-donor starting material. The absence of compensation also helps sponsors and their suppliers to align their standards with those of industry-leading organizations and accreditation agencies such as the Foundation for the Accreditation of Cellular Therapy (FACT) and the World Marrow Donor Association (WMDA). Leveraging uncompensated collection processes also supports protections for donors and patients by promoting safety.
Donor protections are important in the United States, yet certain protections go underrecognized in the American life-science industry. For example, the current regulatory framework includes procedures for reporting patient adverse events to the FDA, but that is not the case for donors. As a result, donor adverse events probably are underreported, making it difficult to assess what improvements could benefit donors.
Another consideration is donor follow-up, which neither US nor UK regulations mandate, but which is required by accrediting agencies such as the WMDA. Sponsors should consider how starting-material collection processes might affect donors and provide appropriate resources (e.g., appointments with counselors and medical professionals) during postdonation care.
GMP and Procurement of Cellular Starting Material
The challenge with implementing GMP globally is that countries follow different standards. Even within the European Union, member states take different approaches to GMP application for procurement of starting material. One example is Germany’s certificate of GMP compliance for collection facilities. It is issued under EU directive 2003/94/EC, which defines GMP principles and guidelines for investigational and medicinal products for human use. But in the rest of the European Union, procurement of starting material does not need to adhere to GMP as long as facilities are inspected regularly or audited by regulatory authorities.
During an FDA Office of Tissues and Advanced Therapies (OTAT) town-hall meeting on 29 September 2022, the agency clarified that, in the United States, GMP does not apply to collection of allogeneic starting material (10). Sponsors must adhere to 21 CFR 1271 subpart C specifically, not to full GMP conditions, which are required at biomanufacturing sites. However, the FDA does recommend that cell-therapy companies implement standard procedures for collecting, storing, and shipping cellular material, especially if collections occur at multiple sites.
The United Kingdom and Canada currently provide no specific requirements for applying GMP during collection processes. GMP is applied once product manufacturing begins.
Best Practices for Navigating Regulatory Complexity
Today, cell-therapy companies lack simple means for assessing donors and collecting cellular starting materials in compliance with multiple global regulations. The best approach for such companies is to understand their international business goals from the start. Then, they can research regulatory considerations in different jurisdictions, compare those regions’ respective requirements for starting-material collection and product distribution, and finally incorporate donor and procurement requirements into their biomanufacturing processes. Harmonization of international regulatory differences will be an important goal in the coming years. With health authorities having taken initial steps in such work, the next few years should bring exciting developments.
References
1 21 CFR 1271. Human Cells, Tissues, and Cellular and Tissue-Based Products. US Code Fed. Reg. 25 May 2004; https://www.ecfr.gov/current/title-21/chapter-I/subchapter-L/part-1271?toc=1.
2 CBER 2004D-0193. Guidance for Industry: Eligibility Determination for Donors of Human Cells, Tissues, and Cellular and Tissue-Based Products. US Food and Drug Administration: Silver Spring, MD, August 2007; https://www.fda.gov/regulatory-information/search-fda-guidance-documents/eligibility-determination-donors-human-cells-tissues-and-cellular-and-tissue-based-products.
3 Guidance Agenda: Guidance Documents CBER Is Planning To Publish During Calendar Year 2023. US Food and Drug Administration: Silver Spring, MD, 2023; https://www.fda.gov/vaccines-blood-biologics/biologics-guidances/guidance-agenda-guidance-documents-cber-planning-publish-during-calendar-year-2023.
4 CBER 2008-D-0205. Chemistry, Manufacturing, and Control (CMC) Information for Human Gene Therapy Investigational New Drug Applications (INDs): Guidance for Industry. US Food and Drug Administration: Silver Spring, MD, January 2020; https://www.fda.gov/regulatory-information/search-fda-guidance-documents/chemistry-manufacturing-and-control-cmc-information-human-gene-therapy-investigational-new-drug.
5 Regulatory Roadmap for Biologic (Schedule D) Drugs in Canada. Health Canada: Ottawa, Canada, 14 December 2022; https://www.canada.ca/en/health-canada/services/drugs-health-products/biologics-radiopharmaceuticals-genetic-therapies/regulatory-roadmap-for-biologic-drugs.html.
6 Cells, Tissues, and Organs. Health Canada: Ottawa, Canada, 22 March 2022; https://www.canada.ca/en/health-canada/services/drugs-health-products/compliance-enforcement/information-health-product/cells-tissues-organs.html.
7 Proposal for a Regulation on Substances of Human Origin. European Commission: Brussels, Belgium, July 2022; https://health.ec.europa.eu/blood-tissues-cells-and-organs/overview/proposal-regulation-substances-human-origin_en.
8 21 CFR 207.13(c). Requirements for Foreign and Domestic Establishment Registration and Listing for Human Drugs, Including Drugs That Are Regulated Under a Biologics License Application, and Animal Drugs, and the National Drug Code. US Code Fed. Reg. 31 August 2016; https://www.ecfr.gov/current/title-21/chapter-I/subchapter-C/part-207.
9 JACIE Accreditation. European Society for Blood and Marrow Transplantation: Madrid, Spain, 2023; https://www.ebmt.org/jacie-accreditation.
10 OTAT Town Hall: Gene Therapy Chemistry, Manufacturing, and Controls. US Food and Drug Administration: Silver Spring, MD, 29 September 2022; https://www.fda.gov/news-events/otat-town-hall-gene-therapy-chemistry-manufacturing-and-controls-09292022.
Beth Kuker, MS, is regulatory affairs manager at Be The Match BioTherapies, 500 North 5th Street, Minneapolis, MN 55401; [email protected]; https://bethematchbiotherapies.com.
You May Also Like






