- Sponsored Content
- Process Development
Mind the Gap: Managing Relationships Between Upstream and Downstream IntensificationMind the Gap: Managing Relationships Between Upstream and Downstream Intensification
Sponsored by Sartorius
 Process intensification (PI) describes an integrated framework of strategies to maximize the output of a unit operation, a process, or an entire facility. By implementing PI strategies, biomanufacturers can accomplish their productivity goals by increasing production speeds and titers, reducing facility footprints, and cutting costs. Overall, such changes improve production efficiency and flexibility.
Process intensification (PI) describes an integrated framework of strategies to maximize the output of a unit operation, a process, or an entire facility. By implementing PI strategies, biomanufacturers can accomplish their productivity goals by increasing production speeds and titers, reducing facility footprints, and cutting costs. Overall, such changes improve production efficiency and flexibility.
Collectively, the biotherapeutic industry has made multiple advancements in intensifying upstream processing. PI strategies include using high-density cell banks, implementing seed-train intensification (n – 1 perfusion), and incorporating the principles of continuous manufacturing (with concentrated fed-batch production and dynamic perfusion technologies). Such intensification approaches deliver increased titers at the end of upstream process steps. However, manufacturers now reach a potential bottleneck in their intensification journey: handling higher masses and concentrations downstream.
Downstream Process Intensification Bottlenecks
To guarantee a smooth transition from upstream to downstream process steps and maximize the potential benefits associated with PI, biomanufacturers must optimize their downstream processes to handle those higher titers.
Uncharted Waters: A number of established intensification schemes work well across upstream bioprocesses (Figure 1) (1). By contrast, downstream PI processes and platforms are less established and can require more work to develop and optimize. However, the biopharmaceutical industry is experiencing a turning point, with advancements in purification technologies and intensification trends, including evolving purification strategies, a move toward single-use manufacturing, and broadened adoption of continuous processing.
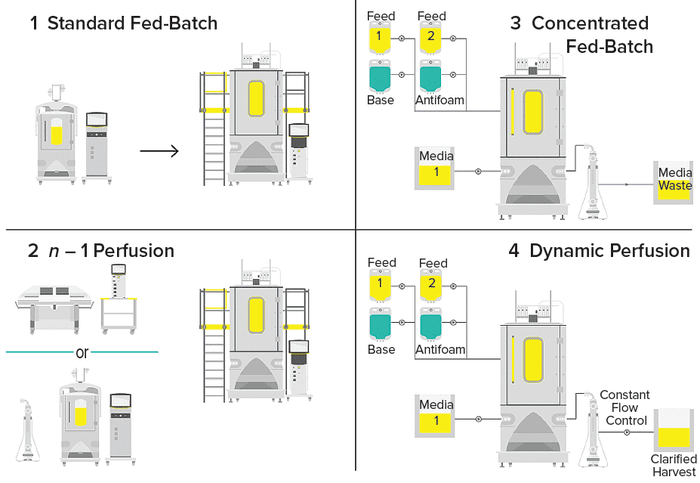
Figure 1: Upstream process-intensification (PI) schemes.
1 The standard cell-culture technique used across the biopharmaceutical industry is fed-batch culture, in which cells are cultured in a bioreactor, typically for 14 days. Protein harvest occurs only at the end of the fed batch. Feed media are added gradually to replenish nutrients and growth factors during the process. | 3 In concentrated fed-batch processes, production and feed media are refreshed continually in a perfusion bioreactor. Spent media pass through a retention device and go to waste. Cells and protein products are recirculated and returned to the bioreactor. The media feed is continuous, but the protein of interest is harvested only at the end of the culture. |
|---|---|
2 Seed-train intensification is one of the quickest and simplest ways to apply process intensification to a new or existing process. In an n – 1 perfusion, highinoculum, fed-batch process, a production bioreactor is seeded with a higher number of cells, reducing the time needed to reach desired cell densities for production or to deliver a higher titer within the same time scale as would a standard fed batch culture. | 4 In perfusion processes, media circulate through a growing culture to supply nutrients while waste is removed and product is harvested in a single operation. The entire volume of medium is exchanged daily while cells are retained and waste is minimized, thus increasing cell density, culture longevity, and protein production. Combined production and feed media are needed to provide sufficient nutrients for high cell densities. |
Fractured Thinking: Implementing up- and downstream PI strategies as individual operations can result in approaches that do not complement one another. At worst, they can be antagonistic. Steps that intensify an upstream process undoubtedly will influence what must be accomplished downstream. Compared with standard fed-batch processes, samples from an intensified upstream process are likely to contain both higher product titers and increased impurities when they move downstream. A biomanufacturer must assess whether its current downstream scheme can handle additional purification challenges. If not, can the facility be modified to adapt to needed changes? Are the required modifications cost-effective?
Unlock Efficiency in Your Downstream Process
To “mind the gap” between upstream and downstream intensification, you need to have detailed insights into the relationships between production stages. When designing your overall strategy for implementing PI, you should consider adopting a stepwise method. Taking a gradual, linear approach can minimize disruptions, reduce development and optimization timelines, and ensure that you can capitalize on the potential benefits of intensification.
Examine Your Existing Process: Before embarking on a PI journey, review your current practices. You may already have adopted some intensification principles: PI need not be an all-or-nothing approach. The broad spectrum of strategies under the PI umbrella includes small, incremental changes to a standard fed-batch process, such as intensifying a seed train (1) or exploring continuous manufacturing strategies (2).
Aim for Synergy: Production steps do not function independently, so you should consider the impact of upstream PI on downstream procedures as soon as possible. For each upstream change, you should examine the effects of increased mass on your downstream process. You should be able to anticipate what will come from the production bioreactor and prepare accordingly — and you will probably need to reoptimize your downstream steps. That will require careful communication and cooperation between departments. In extreme cases, you may need to explore entirely new downstream approaches. For example, increased cell densities generated in a production bioreactor might preclude current clarification procedures from efficiently removing the resulting cell mass. Perfusion/continuous upstream processing requires that you capture your product every day — and ideally, purify it daily. To take full advantage of the benefits of PI upstream, you could implement multicolumn chromatography (MCC), thereby bridging the gap and extending continuous processing strategies across the entire process.
Take a Stepwise Approach: Once you have an idea of operations that can be intensified, you can implement additional intensification steps gradually, starting with upstream and moving into downstream operations. Whether downstream steps require adjustments as a consequence of upstream PI depends on modality and production parameters. A step-by-step strategy ensures that changes are applied as smoothly as possible by considering independent short- and long-term goals. Applying intensification principles might require significant changes to unit operations, equipment, and/or an entire facility. Disruptions should be kept to a minimum to prevent costly delays, which can be an especially critical consideration for small or new facilities.
An Integrated Approach to Process Intensification
Applying PI principles can help biomanufacturers satisfy key business drivers and remain strong in a competitive and tightly regulated market. However, developing a clear integration strategy for upstream and downstream intensification will enable you to reap the rewards of PI.
In an ideal situation, bioprocessors will consider the impacts of their upstream intensification approaches on downstream processes before implementing changes. That provides two related benefits. When considered in the context of upstream production, downstream process steps can be designed to ensure efficient product purification for the titers and volumes generated with PI. And after intensifying upstream production, you can apply similar principles to downstream processing, making your entire production process continuous. As one example, dynamic perfusion culture can be followed by MCC.
Taking a stepwise approach to implementing downstream process intensification and developing a roadmap with clear milestones will help you to reap the rewards of your entire PI journey.
References
1 Matuszczyk J-C, et al. Intensified Seed Train Strategy for Faster, Cost-Effective Scale-Up of Biologics Manufacturing. BioProcess Int. 18(11–12) 2020: 62–64; https://bioprocessintl.com/sponsored-content/rm-perfusion-bioreactors-intensified-seed-train-strategy-for-faster-cost-effective-scale-up-of-biologics-manufacturing.
2 Mihal C. In With the New: Embracing Multicolumn Chromatography. The Medicine Maker (14 May 2021); https://themedicinemaker.com/manufacture/in-with-the-new-embracing-multicolumn-chromatography.
Corresponding author Priyanka Gupta is the head of external collaborations- separations technology at Sartorius; [email protected]; 1-408-872-2442. Katy McLaughlin is a scientific content writer at Sartorius.
You May Also Like






