- Sponsored Content
- Product Characterization
- QA/QC
Mitigate Risk with Effector Function Characterization for Antibody TherapeuticsMitigate Risk with Effector Function Characterization for Antibody Therapeutics
December 4, 2019
Sponsored by Millipore Sigma
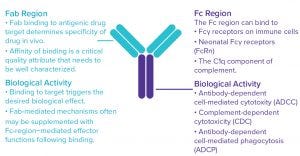
Figure 1: Monoclonal antibodies (MAbs) exert their biological effects through both Fab‑and Fc-mediated activities.
The complexities of biomanufacturing combined with heterogeneity introduced by cellular expression systems present significant challenges to assessing the quality of biologics such as monoclonal antibodies (MAbs). Information related to the critical quality attributes (CQAs) of MAb drug candidates is unknown during early phase drug development. It must be established empirically by physical, structural, and functional analyses as early as possible to accelerate development and mitigate risk through greater understanding of product characteristics.
High-resolution analytical techniques are required to answer questions regarding product heterogeneity with respect to charge, size, glycan structure, and other posttranslational modifications. In addition to those analyses, biological characterization helps scientists answer critical questions about how a drug functions and provides insight into its structure–function relationship.
Investing in select sophisticated analytics in early development can help mitigate risks throughout a product lifecycle by confirming that a MAb candidate has the basic characteristics and functionality required, which can eliminate many unknowns during the development process. Use of key assays provides early information about a product and helps to inform critical decisions at key stages such as clone selection, upstream and downstream process optimization, and determination of CQAs.
Therapeutic antibodies can work in a number of ways. Their clinical efficacy often depends on a combination of biological functions mediated by both the antigen-binding (Fab) and crystallizable (Fc) fragments (Figure 1). Crucial functional characterization information includes how strongly an antibody drug candidate binds its target(s) and to what extent will engage a patient’s immune system to bring about cell-mediated and/or complement-mediated cytotoxic effects (effector functions).
Fab regions bind with high specificity to their antigenic drug targets. That binding alone typically can mediate biological effects such as induction of cell death or cell proliferation. Once an antibody is bound to its target, Fc regions may bind to Fcγ receptors on immune cells or the C1q complement protein, which in turn can bring about additional effector functions. In all such cases, binding affinity is a CQA that must be well characterized, followed by characterization of the functional significance of the binding using sensitive assays to measure antibody- dependent cellular cytotoxicity (ADCC), complement-dependent cytotoxicity (CDC), and/or antibody-dependent cell-mediated phagocytosis (ADCP) activity (Figure 2).
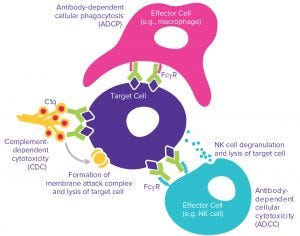
Figure 2: Possible effector functions — antibody-dependent cellular cytotoxcity (ADCC), complement-dependent cytotoxicity (CDC), and antibody-dependent cell-mediated phagocytosis (ADCP)
Combining physical and/or structural binding and biological activity data helps researchers establish whether a product has the necessary attributes for its intended clinical use. For example, knowledge regarding likely effector functionality can be critical to tumor-targeting MAb therapies for which ADCC and/or CDC can be important contributors to overall efficacy. Even if effector functions are not considered to contribute to mechanism of action (MoA), regulatory authorities require an understanding of all possible biological activities because effector functions also can have implications for drug safety. It is therefore critical to characterize all the different biological activities that an antibody mediates.
The extent of characterization of binding and functional activities in early development can differ greatly among drug developers. Such differences may depend on the likely contribution of effector functions to MoA, the level of risk aversion, and availability of resources and technical capabilities. However, for maximum risk mitigation, key binding and functional activities should be assessed as early as possible in development.
By contrast, for a biosimilar MAb, detailed comparison of all binding and functional activities using multiple orthogonal methods is needed as soon as possible in development to assess the molecule’s level of similarity to the originator and satisfy the expectations for comparability testing to enable clinical assessment. Hence, approaches to the extent, timing, and methods used to characterize MAb biological functionality vary. But selective use of the most up-to-date, sensitive methods provides crucial insight to support successful MAb development.
Characterization of Effector Functions
To achieve a level of product understanding that helps ensure quality and enables risk reduction, sensitive cell-based assays that reflect the possible mechanisms of biological activity in vivo are essential. Such assays are used to determine the significance of structural attributes and variability on a drug’s biological function or, in the case of biosimilars, assess the functional significance of differences between molecules.
Fcγ receptor and C1q binding affinities and their effects on ADCC, CDC, and ADCP functions must be tested individually using the most sensitive binding or cell-based assays available. Understanding all the possible biological activities of an antibody enables selection of the most appropriate characterization methods to establish and monitor product CQAs.
Binding Kinetics: Surface plasmon resonance (SPR) has become a leading technology for analyzing both Fab and Fc-based antibody kinetic-binding interactions. This method of kinetic analysis is more informative than enzyme-linked immunosorbent assays (ELISAs) in that it provides real-time association and dissociation rates. SPR and other rapid methods for analysis of binding interactions such as biolayer interferometry (BLI) are invaluable for early product characterization because they are rapid and cost-effective, requiring minimal sample volumes and providing detailed insight about the nature of the binding interaction.
Functional Activity: Evaluating functional activity such as whether an antibody engages immune system components — e.g., natural-killer (NK)cells, complements, and macrophages — to bring about effector functions mitigates the risk of selecting a drug with poor efficacy. Building on insights predictive of likely effector functions gained by Fcγ receptor or C1q binding analysis requires further characterization of effector functions using physiologically relevant cell-based assays to reveal the full story regarding MAb biological activity. However, the cell-based methods used to characterize effector functions can be challenging to perform and require sourcing of primary human blood cells/complement. Consequently, such methods often are outsourced to service providers with experience in establishing and optimizing assays for each MAb.
ADCC assays measure the in vitro cell-killing activity induced by a MAb binding to a target antigen in a suitable cell line. Subsequent binding of primary immune effector cells such as whole peripheral blood mononuclear cells (PBMCs) or purified NK cells to the MAb Fc region (through Fcγ receptors) leads to degranulation of effector cells and destruction of the target cell. CDC assays are less challenging than some others because no primary cells are involved. However, a suitable human complement is a critical reagent that may give rise to variability in an assay to measure the cytotoxic effect resulting from binding of MAb to target cells, followed by Fc-region–mediated binding to C1q. This results in formation of a membrane attack complex (MAC) and lysis of the target cells.
ADC Effector Characterization |
|---|
Understanding effector functions is equally important for other MAb based modalities such as antibody–drug conjugates (ADCs). Although the primary mechanism of action (MoA) for ADCs is direct cytotoxicity through intracellular release of a toxin following specific binding to target tumor antigen, Fc-region–mediated recruitment of immune cells and triggering of effector functions also may be important contributors to overall antitumor efficacy. The harnessing of immune-mediated cell killing might offer obvious benefits, but in some clinical situations, effector function can be an undesirable attribute for the ADCs.The role of the antibody in directing an ADC to tumor cells and the potential for immune recruitment as a contributing MoA underpins the importance of understanding the antibody structure–function relationship and highlights the usefulness of early characterization to inform whether an ADC has the desired quality attributes for its chosen application.Functional characterization of ADCs elucidated by selected binding and sensitive cell-based assays, which assess effector function activity following binding to the target antigen, provides critical insight into overall cytotoxic activity resulting from both direct cytotoxicity and immune-mediated cell killing. For situations in which immune recruitment and one or more effector functions are considered critical quality attributes (CQAs), data from binding and cell-based assays will indicate any possible impact on functionality following conjugation of the chosen linker and toxin combination. Further, those assays could be used to inform decisions on the choice of linker used or the method of conjugation if Fc-mediated binding of Fcγ receptors and one or more effector functions are considered crucial for efficacy and could be impacted by the conjugation process. |
ADCP activity is typically the most technically complex of the three effector functions to characterize in vitro and also requires flow cytometry instrumentation. Target cells that express the target antigen are combined with the antibody and primary phagocytic cells. Primary macrophage-based assays offer greatest sensitivity, but differentiation of macrophages from primary monocytes is labor intensive and time consuming. The required endpoint analysis of phagocytocytic engulfment of the target cells requires labeling of target cells and careful flow cytometry gating and analysis. That will enable researchers to identify subsets of the cell population with both macrophage-specific labeling and target cell dye detection — an analysis that is technically more demanding to optimize than plate-reader–based cell-death endpoints.
In all cases, establishing suitable effector-function assays requires sourcing of target cell lines, primary human blood cells (effector cells), the capability to isolate or differentiate relevant cell types to maximize assay sensitivity, optimization of assay conditions to generate a robust dose-response curve, and a highly sensitive and selective endpoint detection method.
Partnering for Success
Early stage adoption of methods to characterize MAb products can be beneficial at all stages, from clone selection to optimization of a manufacturing process for reducing risk in biologics development and informing decisions during a product lifecycle. An in-depth understanding of product and process design ensures that required safety, purity, identity, and potency profiles are achieved by revealing how the manufacturing process influences those profiles and what levels of product heterogeneity can be tolerated while still maintaining desired functionality.
Characterization of effector function through comprehensive understanding of structural, binding, and biological activity attributes can be challenging. It requires access to specialized analytical methods, advanced instrumentation, and expertise in method development and interpretation. An experienced partner can offer established methodologies and established sources of primary cells as well as capabilities in developing and optimizing assay methods for multiple diverse MAb products and for interpreting data from effector function assays. Such a partner not only can perform these challenging cell-based assays, but also will offer the expertise needed to combine data from other analyses (e.g., glycosylation results and binding data from SPR) and holistically interpret those data to determine structure–function relationships.
A broadly capable partner provides a combined offering of physicochemical, structural, and functional characterization using orthogonal approaches to simplify product characterization. That will give a product sponsor confidence by providing data to ensure that a product has the relevant quality attributes for its intended use. Additional benefits of working with an outsourced partner can arise from tapping into experience gained in selecting the most insightful methods to implement in early stages — in particular, to maximize the value of characterization data generated when full characterization is not appropriate.
In addition, using already established methods, experience, and interpretation expertise can accelerate product characterization, which demands a significant investment of resources and time. Service providers with options available for analysis of structural and functional attributes, offer a number of approaches and flexibility that can be tailored to a drug developer’s needs and time constraints, budget, and level of insight required. In addition to the most physiologically relevant or high-resolution assays, rapid and cost-effective approaches suitable for use in early stages typically offer insight into functional activity and structure–function relationship to support product development. Examples include use of rapid glycan profiling methods such as apillary electrophoresis-laser-induced fluorescence (CE-LIF), rapid SPR or BLI analyses of binding interactions using preestablished assay methods, and analysis of effector functions using surrogate reporter cell-based assays.
Pamela Hamill, PhD, is principal scientist, research and development services, MilliporeSigma ([email protected]). The life science business of Merck KGaA, Darmstadt, Germany, operates as MilliporeSigma in the United States and Canada.
You May Also Like






