- Sponsored Content
Navigating Diverse Production Challenges: Four Routes to SuccessNavigating Diverse Production Challenges: Four Routes to Success
Sponsored by Sartorius
The biopharmaceutical industry is facing continued pressures to bring high-quality drugs to the market quickly while limiting costs and meeting challenging sustainability targets. However, companies might lack the expertise, resources, and time required to establish, optimize, and scale up tailored, efficient processes that meet market demands.
Process consultancy services and preengineered solutions are excellent resources that bring in new perspectives and expertise, regardless of your specific end goals. Here, we outline four key objectives, the challenges associated with meeting them, and a suitable service for each objective to help meet your needs.
Objective #1: Increase Output While Minimizing Costs
With the “patent cliff” approaching, biopharmaceutical companies already in commercial manufacturing must cope with increasing market pressure from biosimilar competitors. Therefore, maximizing process efficiency is critical. That includes increasing batch numbers and production yields while reducing manufacturing costs to improve overall facility use.
Facility footprint limitations and the burdens of validating new technologies and regulatory compliance often hinder process improvements. Legacy processes can yield variable titers from upstream process steps that increase risks of batch contamination from outdated technology. In-depth process understanding and expertise in facility design, process modeling, and equipment limitations are required to build an efficient process.
These features are offered by the Sartorius Value Chain service (Figure 1).
The Sartorius Value Chain service applies the company’s collective process and product expertise to help clients improve operational efficiency and reduce or delay capital investment for commercial processes. The service offers comprehensive strategies for process scale-up, process improvements, and technology-transfer projects.
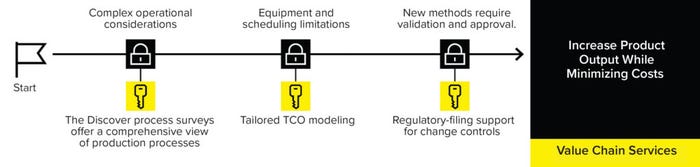
Figure 1: The Sartorius Value-Chain services offer comprehensive strategies for process scale-up, improvements, and tech-transfer projects.
Sartorius experts first will carry out a Discover process survey informed by a “gemba walk” — Japanese for actual place — to gain a comprehensive view of a client’s end-to-end production process, identifying the most valuable improvement opportunities in a complex process. The team will use that information to help clients select the right equipment, set-up, and process schedule to minimize their total cost of ownership (TCO). The time-consuming process of validating new methods might discourage biomanufacturers from making changes; however, the Sartorius Value Chain services provide support for change controls necessitated by implementation of new technologies or methods.
Objective #2: Build a New and Adaptable Facility
Biopharmaceutical companies and contract development and manufacturing organizations (CDMOs) often operate complex projects and processes simultaneously. The increasing diversity of biopharmaceutical modalities is likely to make that process more challenging. New facilities need to be streamlined, cost-effective, and flexible to adapt to changing customer needs.
Waiting to consider equipment and consumables until after facility completion can lead to ill-fitting technologies or the need to customize technologies completely, increasing costs, creating longer lead times, and decreasing assurance of supply. To reduce complexity in long-term operations, manufacturing rooms must be set up to maximize their use of space. Applying a flexible set of “standardized” consumables simplifies operations and ensures consistent supply. The Sartorius Integrated Solutions (InSo) consulting services take a process-first approach, tailoring end-to-end single-use (SU) manufacturing solutions from concept to realization (Figure 2).
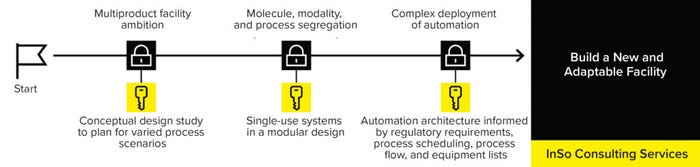
Figure 2: The Sartorius InSo process-first services tailor end-to-end single-use (SU) manufacturing solutions from concept to realization.
The InSo team collaborates with biomanufacturers and good manufacturing practice (GMP) facility-design partners to develop tailored implementation methodologies for clients’ bioprocesses, using SU technologies wherever possible. Implementing SU systems in a modular design can help improve flexibility and limit downtime, especially in multiproduct facilities. Such modern layouts operate with limited fixed equipment, and processes are segregated by use of automation and closed systems.
InSo consulting performs early evaluations of key process, regulatory, and facility design variables and helps clients strategize the adoption of SU technologies and intensification strategies to create a flexible modular facility for the dynamic biopharmaceutical landscape. This includes how SU technologies will be implemented, e.g., by selecting end-to-end SU platforms or identifying opportunities for SU solutions in a hybrid facility.
Objective #3: Expand Quickly into Emerging Markets
Providing fast, cost-effective access to medicines in underserved markets is an important driver for many biopharmaceutical companies. However, it requires rapid facility set-up, equipment installation, and process qualification, often in locations with limited existing infrastructure.
Further confounding this challenge, the market outlook forecasts the emergence of new underserved markets in five to 10 years. Thus, facilities must be flexible enough to cope with changing demands and expand their capacity when needed, all while complying with sustainability targets. Sartorius’s rapid-deployment services offer preengineered solutions to support manufacturers in supplying drug products to patients in need (Figure 3).
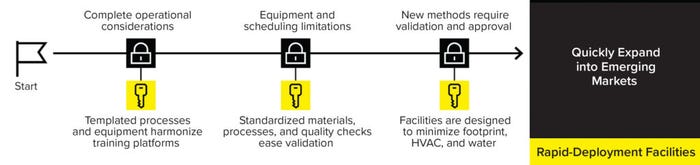
Figure 3: The Sartorius Rapid-Deployment services offer preengineered solutions to support manufacturers.
Rapid-deployment facilities are modular solutions that drive consistency across sites to ensure fast qualification. These sites adhere to a standardized design of prefabricated cleanrooms and laboratories that house equipment and consumables from templated production processes. That enables quick and cost-effective construction of smaller-footprint facilities.
The prefabricated solutions expedite process set-up, limiting time-consuming validation efforts and factory acceptance testing. Also, templated production processes and equipment harmonize training platforms. That enables the exchange of talent between sites, a particularly valuable feature in emerging markets, in which a lack of infrastructure and sparse talent pool might form roadblocks to an efficient manufacturing process. Finally, rapid-deployment facilities have a small footprint and consume less energy, supporting sustainability and cost-saving initiatives.
Objective #4: Scale Up to Pilot Production
Companies in late-phase clinical development need to scale up their process to pilot production in support of commercialization. However, they are likely to have minimal time and resources to invest in engineering and process development. Manufacturing plans must be agile to cope with changing demands such as for new products or adoption of intensified processing. A preengineered, end-to-end process approach — offered through the Sartorius ProcessGo solution — can accelerate scale-up to commercial production and minimize risks (Figure 4).
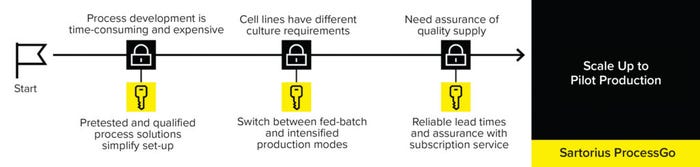
Figure 4: The Sartorius ProcessGo preengineered, end-to-end process solution accelerates scale-up and minimizes risks.
The Sartorius ProcessGo solution is a scalable, preengineered, fully aseptic, and automated platform designed to simplify logistics and limit the risk of project delay. Ready-to-connect and prequalified equipment, consumables, and software support management of fed-batch–based culture, clarification, and media, with the flexibility to intensify the n – 1 seed step, accommodating different cell line requirements.
The Sartorius ProcessGo approach is completed by training from application specialists, installation and implementation support, and supply assurance, with subscription-service contracts for consumables.
Diverse Business Services, Diverse Solutions
Biopharmaceutical companies have diverse business drivers spurred on by factors such as development phase, product type, production scale, and region. As such, it is unrealistic to expect one external service or solution to be suitable for all programs.
However, through our years of experience and success, Sartorius experts have developed a shortlist of services that collectively solve a broad array of bioprocessing challenges. The paths charted illustrate how these services can help you achieve your business goals.
Katy McLaughlin is a scientific content writer. Corresponding author Charles Meadows is a process technology manager at Sartorius; [email protected]. For more information about Sartorius Integrated Solutions (InSo) services, please visit https://www.sartorius.com/en/services/bioprocess-development-engineering. To read more about Sartorius ProcessGo services, please visit https://www.sartorius.com/en/pr/processgo.
You May Also Like






