- Sponsored Content
Predicting Viral Clearance at Your BenchtopPredicting Viral Clearance at Your Benchtop
August 10, 2020
Sponsored by Cygnus Technologies
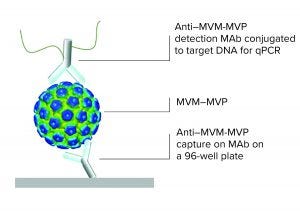
Figure 1: Immuno qPCR
Viral contamination is an inherent risk during the manufacture of therapeutic products such as antibodies, vaccines, viral vectors, and plasma derivatives. Whether introduced endogenously from raw materials or exogenously through manufacturing operations, unmitigated viral contaminations can lead to serious health implications and facility shutdowns. Thus, international regulatory agencies require sponsoring companies to validate the “viral clearance efficacy” of their downstream purification process steps before clinical trials or commercial approval.
This technology review describes the MockV MVM kit for viral clearance prediction. Fill out the form below to read the complete article now.
You May Also Like






